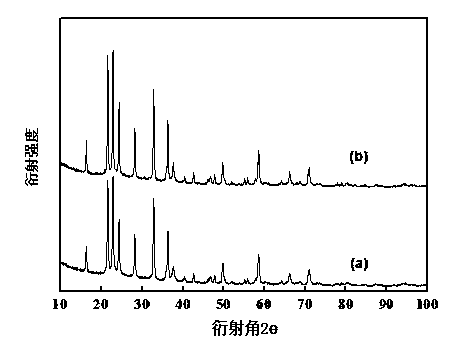
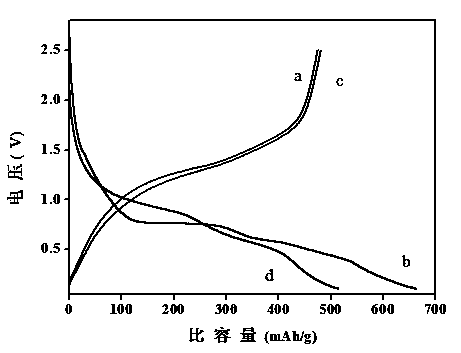
Sol-gel preparation method of lithium vanadate negative electrode material of lithium ion battery
A battery lithium vanadate and negative electrode material technology, applied in battery electrodes, secondary batteries, chemical instruments and methods, etc., can solve the problems of no obvious improvement in electrical conductivity, low intrinsic conductivity of lithium vanadate, etc., and achieve improved electrical conductivity. Chemical properties, good cycle performance, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Dissolve 3.091g of lithium acetate in 60mL of distilled water, and add 0.918g of vanadium pentoxide powder and 2.112g of citric acid in sequence under magnetic stirring to form a light blue solution. The amount of citric acid is added according to the ratio of citric acid: vanadium = 1:1, and other substances are added according to the stoichiometric ratio Li:V = 3:1. Place the mixed solution in a constant temperature water bath at 80°C and gradually evaporate the water to dryness under the condition of magnetic stirring. After forming a xerogel, put it in a vacuum drying oven at 80°C to continue drying the water completely. Then the dried samples were put into ceramic crucibles and placed in a quartz tube furnace at 30% H 2 + 70% Ar (volume fraction ratio) pre-fired at 400°C for 4 hours in a reducing atmosphere, after cooling, take it out and grind it carefully, then treat it at 650°C for 6 hours under the same atmosphere conditions, and obtain a carbon-coated lithium...
Embodiment 2
[0038] Dissolve 1.131g of lithium carbonate in 60mL of distilled water, add 0.918g of vanadium pentoxide powder and 2.112g of citric acid in turn under the condition of magnetic stirring, and finally form a blue solution with the generation of a large number of bubbles. The amount of citric acid is added according to the ratio of citric acid: vanadium = 1:1, and other substances are added according to the stoichiometric ratio Li:V = 3:1. Place the mixed solution in a constant temperature water bath at 75°C and gradually evaporate the water to dryness under the condition of magnetic stirring. After forming a xerogel, put it in a vacuum drying oven at 90°C to continue drying the water completely. Then put the dried sample into a ceramic crucible, put it into a quartz tube furnace, and pre-fire it at 350°C for 4 hours under a nitrogen atmosphere, take it out after cooling, grind it carefully, and then treat it at 800°C for 6 hours under the same atmosphere condition, and cool it n...
Embodiment 3
[0040] Dissolve 1.259g of lithium hydroxide in 60mL of distilled water, and add 0.918g of vanadium pentoxide powder and 1.820g of glucose in sequence under magnetic stirring to form a blue solution. Among them, the amount of glucose is added according to the ratio of the amount of substance glucose: vanadium = 1:1, and other substances are added according to the stoichiometric ratio Li:V = 3:1. Place the mixed solution in a constant temperature water bath at 70°C and gradually evaporate the water to dryness under the condition of magnetic stirring. After forming a xerogel, put it in a vacuum drying oven at 90°C to continue drying the water completely. Then put the dried sample into a ceramic crucible, put it into a quartz tube furnace, and pre-fire it at 350°C for 4 hours under a nitrogen atmosphere, take it out after cooling, grind it carefully, and then treat it at 500°C for 6 hours under the same atmosphere condition, and cool it naturally Finally, a carbon-coated lithium v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com