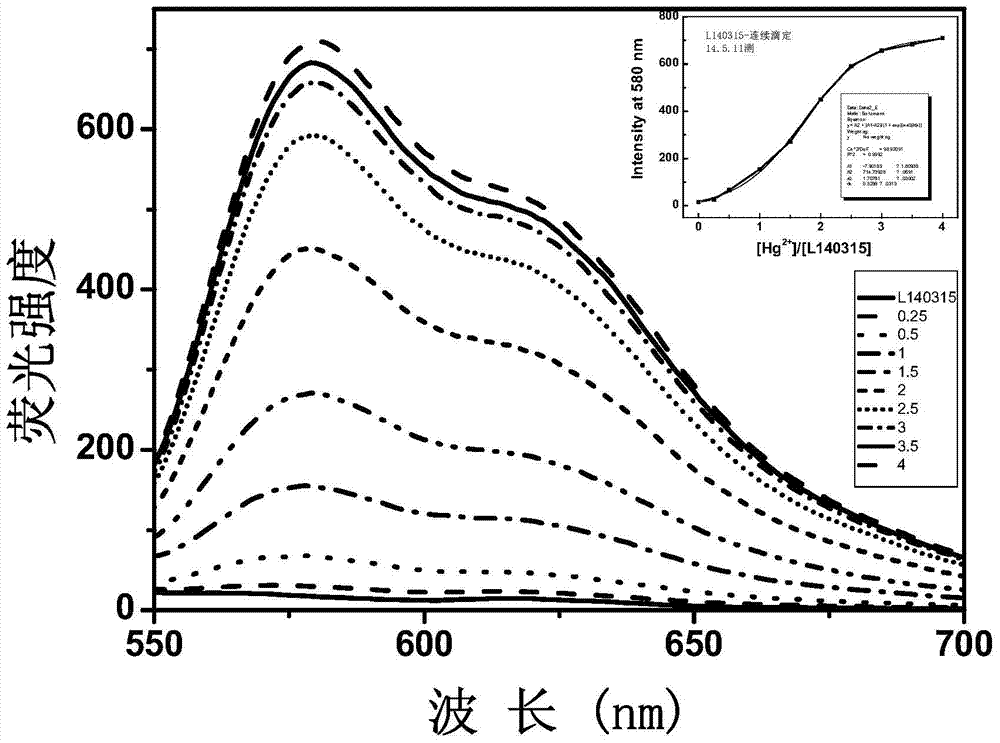
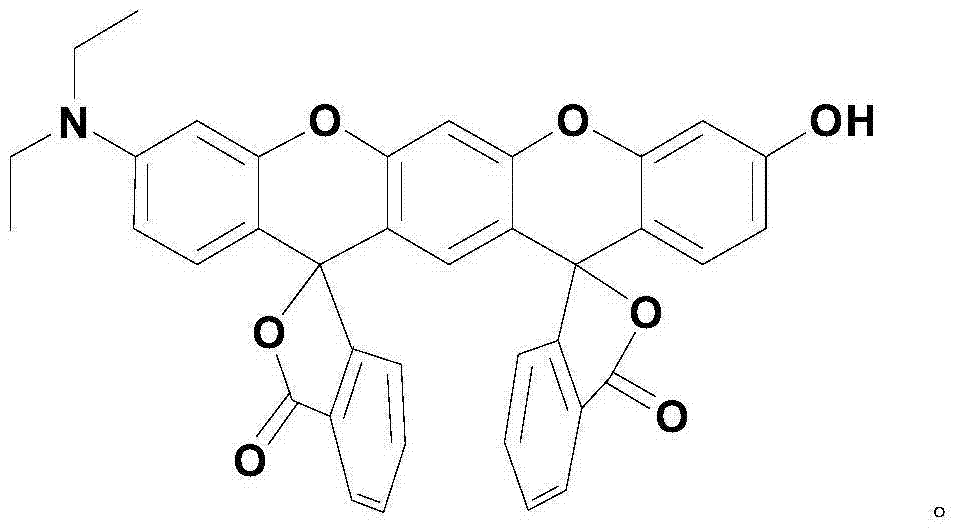
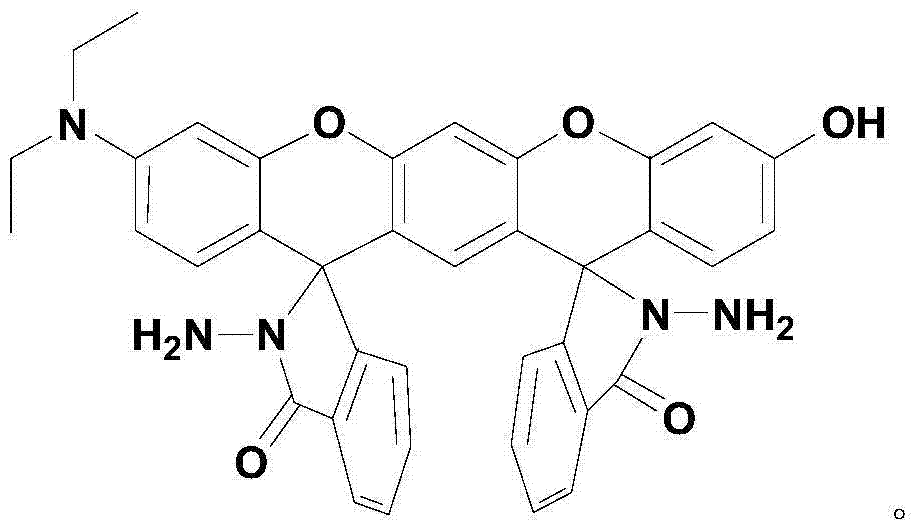
Xanthene fluorescence dye, preparation method and applications thereof
A fluorescent dye, xanthene technology, applied in the field of fluorescence sensing and detection, can solve the problems of reduced fluorescence quantum yield, poor specific selectivity, pH value sensitivity, etc., and achieves high selectivity, low cost, and wide pH value range. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1, preparation of xanthene fluorescent dye molecular probe
[0040] The specific method is as follows:
[0041] The xanthene fluorescent dye molecular probe is 3',3''-bis(oxospiroisobenzofuran)-3-hydroxyl-7-(diethylamino)benzopyran-xanthene, the preparation steps as follows:
[0042] 1) Add 2-(4-diethylamino-2-hydroxybenzoyl)benzoic acid (313 mg, 1 mmol) and fluorescein (332 mg, 1 mmol) into concentrated sulfuric acid (8 mL) and stir. Protect from light and heat the oil bath at 95°C, and react for 5 hours;
[0043] 2) The above reaction solution was cooled to room temperature, poured into 80 mL of ice water and stirred, and the pH of the reaction solution was adjusted to neutral with 1.0 M sodium hydroxide aqueous solution. Then, the mixed solution was extracted several times with dichloromethane (50 mL). The collected organic phase was dried with anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure to obtain a crude produ...
Embodiment 2
[0046] Embodiment 2, preparation of xanthene fluorescent dye molecular probe
[0047] The specific method is as follows:
[0048] The xanthene fluorescent dye molecular probe is 3',3''-bis(oxospiroisobenzofuran)-3-hydroxyl-7-(diethylamino)benzopyran-xanthene, and the steps are as follows :
[0049] 1) Add 2-(4-diethylamino-2-hydroxybenzoyl)benzoic acid (344 mg, 1.1 mmol) and fluorescein (398 mg, 1.2 mmol) into concentrated sulfuric acid (10 mL) and stir. Protect from light and heat the oil bath at 98°C, and react for 4 hours;
[0050] 2) The above reaction solution was cooled to room temperature, poured into 80 mL of ice water and stirred, and the pH of the reaction solution was adjusted to neutral with 1.0 M sodium hydroxide aqueous solution. Then, the mixed solution was extracted several times with dichloromethane (50 mL). The collected organic phase was dried with anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure to obtain a crude pro...
Embodiment 3
[0053] Embodiment 3, preparation of xanthene fluorescent dye molecular probe
[0054] The specific method is as follows:
[0055] The xanthene fluorescent dye molecular probe is 3',3''-bis(oxospiroisobenzofuran)-3-hydroxyl-7-(diethylamino)benzopyran-xanthene, and the steps are as follows :
[0056] 1) Add 2-(4-diethylamino-2-hydroxybenzoyl)benzoic acid (312 mg, 1 mmol) and fluorescein (365 mg, 1.1 mmol) into concentrated sulfuric acid (12 mL) and stir. Protect from light and heat the oil bath at 100°C, and react for 3 hours;
[0057] 2) The above reaction solution was cooled to room temperature, poured into 80 mL of ice water and stirred, and the pH of the reaction solution was adjusted to neutral with 1.0 M sodium hydroxide aqueous solution. Then, the mixed solution was extracted several times with dichloromethane (50 mL). The collected organic phase was dried with anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure to obtain a crude prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com