A novel synthesis process of ubenimex
A technology of Ubenimex and synthesis process, which is applied in the field of new synthesis process of Ubenimex, can solve the problems of high activity of protective agent, low purity and yield, complicated operation, etc., and achieve easy large-scale industrial production and increase yield. efficiency and purity, and the effect of reducing safety risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
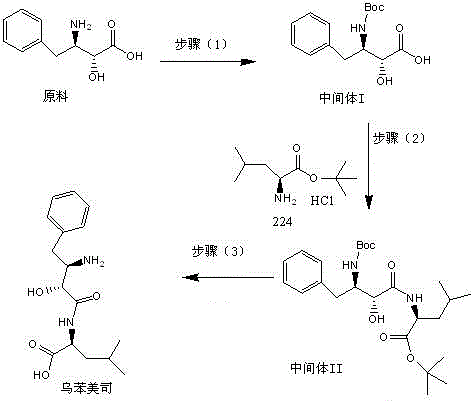
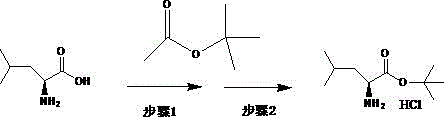
[0037] Preparation of Intermediate I
[0038]Add 15g of raw material (2S, 3R)-3-amino-2-hydroxy-4-phenylbutyric acid and 150mL of water to adjust the pH to 8.5 by adding 2mol / L aqueous sodium hydroxide solution dropwise at room temperature. The system is dissolved and clarified. Add 15ml Acetone and 5g di-tert-butyl dicarbonate, stir for 10 minutes and add 2mol / l sodium hydroxide aqueous solution to adjust the pH to 8.5, then add 5g di-tert-butyl dicarbonate, add 2mol / L hydrogen after stirring for 10 minutes Adjust the pH to 8.5 with aqueous sodium oxide solution, add di-tert-butyl dicarbonate 4 times in total, 20 g in total, continue to stir for 2 hours until the reaction of the raw materials is complete, adjust the pH to 1.5 with 2 mol / L hydrochloric acid in an ice bath, and use 150 ml of ethyl acetate Extract twice, wash the ethyl acetate phase with 100ml saturated brine, dry over anhydrous sodium sulfate, concentrate, add 50mL of petroleum ether to crystallize, filter to o...
Embodiment 2
[0048] Preparation of Intermediate I
[0049] Add 15g of the raw material (2S, 3R)-3-amino-2-hydroxy-4-phenylbutyric acid, 150ml of water at room temperature, add dropwise 2mol / L sodium carbonate aqueous solution to adjust the pH to 9, the system is dissolved and clarified, and add 15ml of methanol and 5g di-tert-butyl dicarbonate, stirred for 10 minutes and added 2mol / l sodium carbonate aqueous solution to adjust the pH to 8.5, then added 5g di-tert-butyl dicarbonate, and added 2mol / l sodium carbonate aqueous solution after stirring for 10 minutes Adjust the pH to 8.5, add di-tert-butyl dicarbonate 4 times in total, a total of 20g, continue to stir for 2 hours until the raw materials are completely reacted, adjust the pH to 2 with 2mol / L hydrochloric acid in an ice bath, and extract twice with 150ml ethyl acetate , the ethyl acetate phase was washed with 100ml saturated brine, dried over anhydrous sodium sulfate, added 50ml petroleum ether to crystallize after concentrating, ...
Embodiment 3
[0056] Preparation of Intermediate I
[0057] The raw material (2S, 3R)-3-amino-2-hydroxy-4-phenylbutyric acid 15g, 150ml of water was added dropwise at room temperature with triethylamine to adjust the pH to 10, the system was dissolved and clarified, and 15ml of triethylamine and 5g Di-tert-butyl dicarbonate, stirred for 10 minutes and added triethylamine to adjust the pH to 8.5, then added 5g of di-tert-butyl dicarbonate, stirred for 10 minutes, added triethylamine to adjust the pH to 8.5, added 4 times in total Di-tert-butyl carbonate, a total of 20g, continue to stir for 2 hours until the raw materials are completely reacted, adjust the pH to 2 with 2mol / L hydrochloric acid under ice bath, extract twice with 150ml ethyl acetate, wash the ethyl acetate phase with 100ml saturated saline , dried over anhydrous sodium sulfate, concentrated, added 50ml of petroleum ether to crystallize, and filtered to obtain a solid, Intermediate I: 21.00g, appearance as white or off-white cr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical rotation | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com