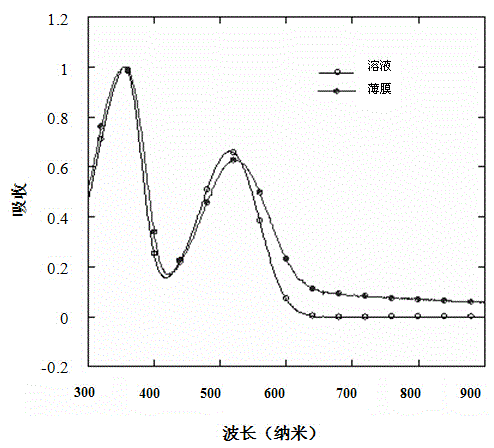
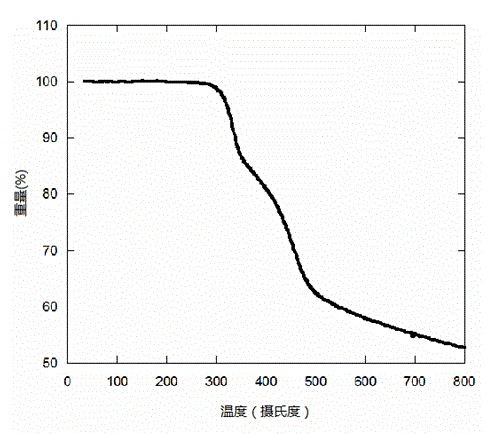
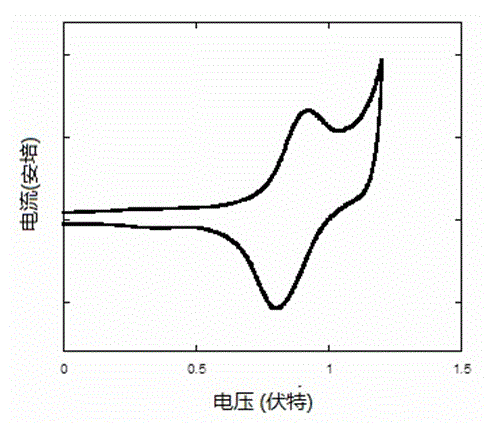
Closed-loop triphenylamine derivative copolymer and preparing method and application thereof
A kind of copolymer and triphenylamine technology, applied in the field of polymer photovoltaics, can solve the problems of unfavorable charge separation, the energy conversion efficiency of solar cells cannot be improved, and achieve lower energy bandgap, excellent photovoltaic performance, and increased spectral absorption range. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of Methyl 2-iodobenzoate
[0033]
[0034] Methyl anthranilate (30.2g, 200mmol) and concentrated hydrochloric acid (36%, 35ml) were successively added to 100ml of distilled water, and stirred for 15min under an ice bath. Slowly add an aqueous solution of sodium nitrite (27.6g, 400mmol), stir in an ice bath for 1 hour, then slowly add an aqueous solution of potassium iodide (90g, 600mmol), control the reaction temperature at 5-8°C, and react overnight in the dark. The reaction solution was extracted with 200ml of dichloromethane, the extract was washed 3 times with saturated sodium chloride solution, and the extract was dried with magnesium sulfate. Dichloromethane was distilled off under reduced pressure, and the initial product was chromatographed on a silica gel / petroleum ether column to obtain 42 g of a colorless liquid with a yield of 80.5%.
Embodiment 2
[0036] Synthesis of 2,2',2"-trimethylcarboxymethyl triphenylamine
[0037]
[0038] Methyl o-iodobenzoate (42g, 161mmol), methyl anthranilate (8.2g, 54mmol), potassium carbonate (18g, 13mmol), copper powder (0.75g, 11.4mmol), cuprous iodide (1.05 g, 5.45 mmol) was added into 300 ml of phenyl ether, nitrogen gas was passed for 5 min, and the reaction was heated and stirred at 190° C. for 48 hours. After cooling, filter with suction, and distill the filtrate to remove the solvent under reduced pressure to obtain a crude product, which is recrystallized from ethanol to obtain 16.97 g of yellow crystals with a yield of 75%.
Embodiment 3
[0040] Synthesis of 2,2',2"-tris(bis(4-methylphenyl)hydroxymethyl)triphenylamine
[0041]
[0042]Dissolve p-bromotoluene (9.6 g, 56 mmol) in anhydrous tetrahydrofuran, and stir at -78°C for 15 minutes under nitrogen protection. Add n-butyllithium (22.5ml, 2.5M, 56.25mmol) dropwise to the p-bromotoluene solution, and stir at -78°C for 1 hour. Add 2,2',2"-trimethylcarboxymethyl triphenylamine (2.1g, 5mmol) in tetrahydrofuran solution dropwise, slowly rise to room temperature, and react overnight. Quench with an appropriate amount of saturated ammonium chloride aqueous solution, and distill under pressure to remove tetrahydrofuran The resulting yellow solid was added to 50ml of ethanol, heated to boiling, and stirred for 0.5 hours. After cooling, it was suction filtered, and the solid was washed once with water, ethanol, and petroleum ether, and dried to obtain 3.9 g of a yellow-green solid with a yield of 89%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com