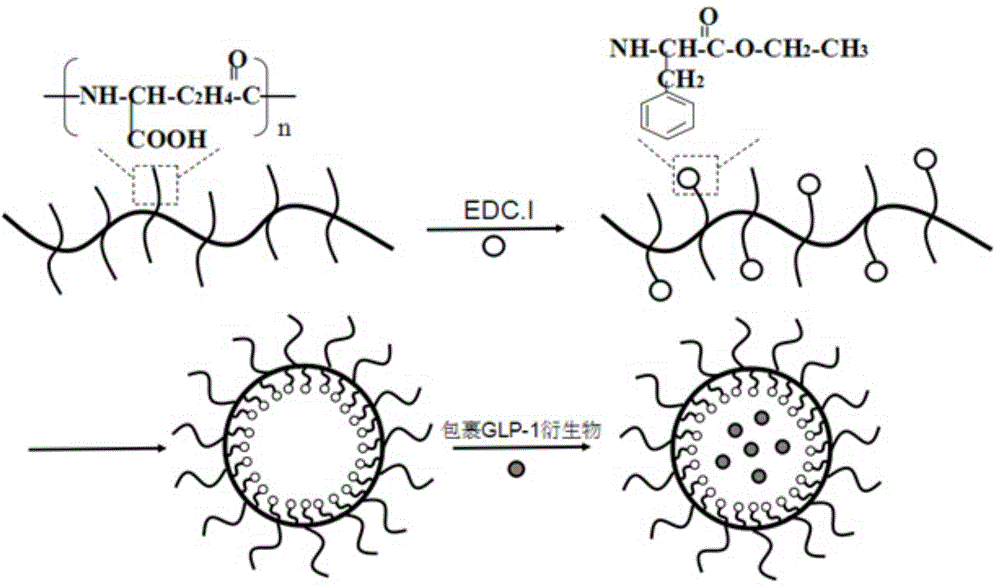
GLP-1 derivative particle compound as well as preparation method and application thereof
A technology of GLP-1 and particle complexes, applied in the field of biopharmaceuticals, can solve the problems of limiting the breadth and depth of use, and achieve the effects of reducing the number of injections, avoiding low encapsulation rate and high encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation method of embodiment 1γ-PGA
[0043] (1) Preparation of γ-PGA
[0044] Strain activation: Take the frozen Bacillus licheniformis ATCC 9945a out of the -80°C refrigerator, and after thawing, insert 1% of the inoculum into the seed medium, and culture at 37°C and 210r / min for 11 hours. Seed medium (LB liquid medium): peptone 1%, NaCl 0.5%, yeast extract 0.5%.
[0045] Fermentation culture: Add the above seed liquid into the fermentation medium according to the inoculum amount of 5%, and cultivate at 37°C and 210r / min for 12h 60-72h until the culture liquid becomes viscous. Fermentation medium: maltose 5%, sodium chloride 1%, yeast powder 1%, sodium glutamate 3%, potassium dihydrogen phosphate 0.5%, magnesium sulfate heptahydrate 0.05%.
[0046] Extraction and purification of γ-PGA: adjust the pH value of the above fermentation culture liquid to 3.0, centrifuge at 10,000 r / min for 30 min to remove bacteria, obtain supernatant, and adjust the pH value to 7...
Embodiment 2
[0049] The preparation of embodiment 2γ-PGA-L-PAE
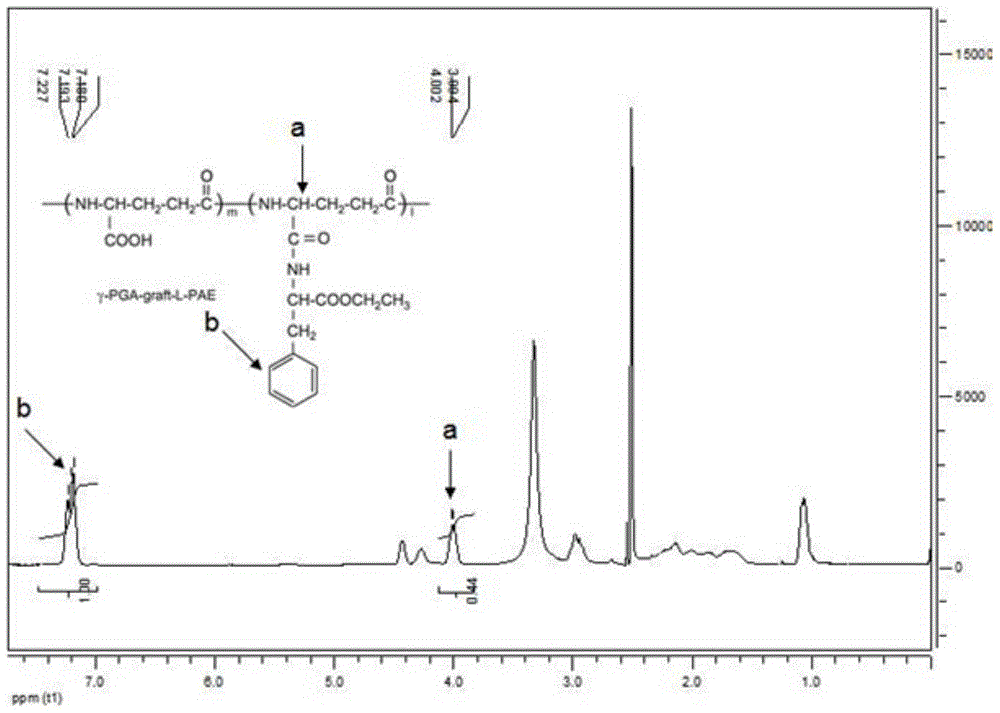
[0050] Weigh 0.1 g of γ-PGA obtained in Example 1 and dissolve it in 50 ml of pure water to prepare a 2% solution, add 2.97 g of EDC.I, shake in a shaker at 37°C and 210 rpm for 15 min, then weigh L- Add 0.96-1.69g of PAE into the reaction system, and continue to react in a shaker at 37°C and 210rpm for 24 hours. A white precipitate was produced in the solution, centrifuged, discarded the supernatant, washed again with ultrapure water, centrifuged and discarded the supernatant, repeated 3 times, and the obtained white precipitate was freeze-dried for 48-72 hours, and the obtained white solid was γ-PGA-L-PAE. For the obtained γ-PGA-L-PAE, carry out proton nuclear magnetic resonance spectrum detection, the solvent is deuterated DMSO, and the spectrum is as follows image 3 As shown, the results show that the characteristic peak of γ-PGA—chemical shift 4.0, and the characteristic peak of L-PAE—chemical shift 8.0 appear in the ...
Embodiment 3
[0051] Preparation and characterization of embodiment 3aGLP-1-NPs
[0052] Preparation method: Dissolve γ-PGA-L-PAE obtained in Example 2 in DMSO to form a 5-40 mg / ml solution. Take 500ul of γ-PGA-L-PAE, slowly add it dropwise to 500ul 0.25~3mg / ml aGLP-1 derivative solution to form a white milky system, centrifuge in a high-speed refrigerated centrifuge at 16000rpm, 4°C for 15min, discard the supernatant , add water again, wash and centrifuge, repeat 3 times, the white precipitate obtained is aGLP-1-NPs, ie aGLP-1-NPs. Wherein, the sequence of aGLP-1 is shown in SEQ ID No:1.
[0053] The aGLP-1-NPs were stained with 1% ammonium molybdate, and the microstructure of the aGLP-1-NPs was observed by transmission electron microscopy, showing a state of round particles with a scale of 100-150nm and uniform distribution, such as Figure 4 Shown; Detected its particle size with a nanometer particle size analyzer, the particle diameter is 140nm, and is a monodisperse system, such as ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com