Functional diamine monomers having high planarity and containing naphthaline structure and synthesis method and application thereof
A technology of diamine monomer and synthesis method, which is applied in the field of material science to achieve the effect of compact packing, easy purification and small free volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of 4,4'-(naphthalene-2,7-diyl)dianiline:
[0040]
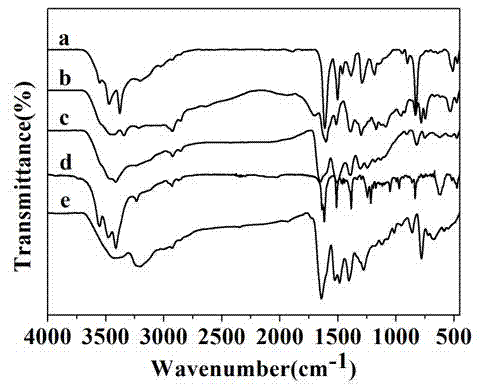
[0041] Add 8.579g (0.03mol) of 2,7-dibromonaphthalene and 11.272g (0.065mol) of p-aminophenyl borate hydrochloride into a 500ml three-necked flask, add 400ml of tetrahydrofuran, and then add 2mol / L potassium carbonate solution 97.5ml and an appropriate amount of Aliquat336, magnetically stirred and argon, heated to 75°C in an oil bath, added 0.100g tetrakistriphenylphosphinepalladium, and refluxed for 24h. The reaction solution was poured into water, and a large amount of precipitates precipitated out. Suction filter with a funnel, evaporate the solvent under reduced pressure. The product was purified by column chromatography using dichloromethane as the mobile phase and silica gel as the stationary phase. The product was collected and spin-dried to obtain an orange-red solid, which was vacuum-dried at 60° C. for 24 h with a yield of 80%. The infrared spectrum of the compound is shown in figure 1 As sho...
Embodiment 2
[0043] Synthesis of Bis(4-aminobenzyl)naphthalene-2,6-dicarboxylate:
[0044]
[0045] (1) Synthetic intermediate naphthalene-2,6-dicarbonyl dichloride
[0046] 10.810g (0.050mol) of 2,6-naphthalene dicarboxylic acid was added to a 250ml three-necked flask, 100ml of dehydrated dichloromethane was added, and 17.846g (0.150mol) of thionyl chloride was slowly added dropwise under ice bath conditions. Add 3 to 4 drops of N,N-dimethylformamide dropwise as a catalyst, stir magnetically and flow argon, heat up to 75°C and reflux for 12h. The solvent and excess thionyl chloride were distilled off under reduced pressure to obtain a light yellow solid with a yield of 85%. The intermediate structure is as follows:
[0047]
[0048] (2) Synthesis of intermediate bis(4-nitrobenzyl)naphthalene-2,6-dicarboxylate:
[0049] Add 18.376g (0.12mol) of 4-nitrobenzyl alcohol into a 500ml three-necked flask, add 200ml of N,N-dimethylacetamide, 40ml of triethylamine, and slowly add the napht...
Embodiment 3
[0054] N 2 ,N 6 Synthesis of -bis(4-((4-aminophenyl)amino)phenyl)naphthalene-2,6-dicarboxami-de:
[0055]
[0056] (1) synthetic intermediate naphthalene-2,6-dicarbonyl dichloride according to Example 2;
[0057] (2) Synthetic intermediate N 2 ,N 6 -bis(4-aminophenyl)naphthalene-2,6-dicarboxamide:
[0058] Dissolve 10.814g (0.1mol) of p-phenylenediamine in a 4:1 solution of N-methylpyrrolidone and pyridine in 150ml, then slowly add 5.061g (0.02mol) of naphthalene-2,6-dicarbonyl dichloride under argon Add 12.420g (0.04mol) of triphenyl phosphite under ambient conditions, stir at room temperature for 2 hours, then raise the temperature to 100°C for 12 hours, pour the reaction solution into methanol after cooling, filter out the precipitate, wash thoroughly with methanol, - Recrystallize from dimethylformamide and water, dry in a vacuum oven at 50°C for 10 hours to obtain off-white product N 2 ,N 6 -bis(4-aminophenyl)naphthalene-2,6-dicarboxamide 6.739 g, yield 85%. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com