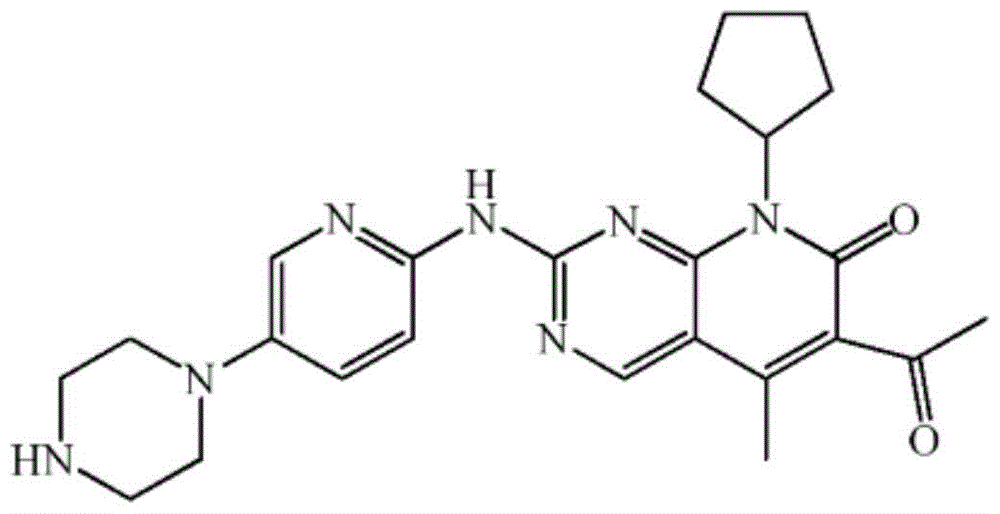
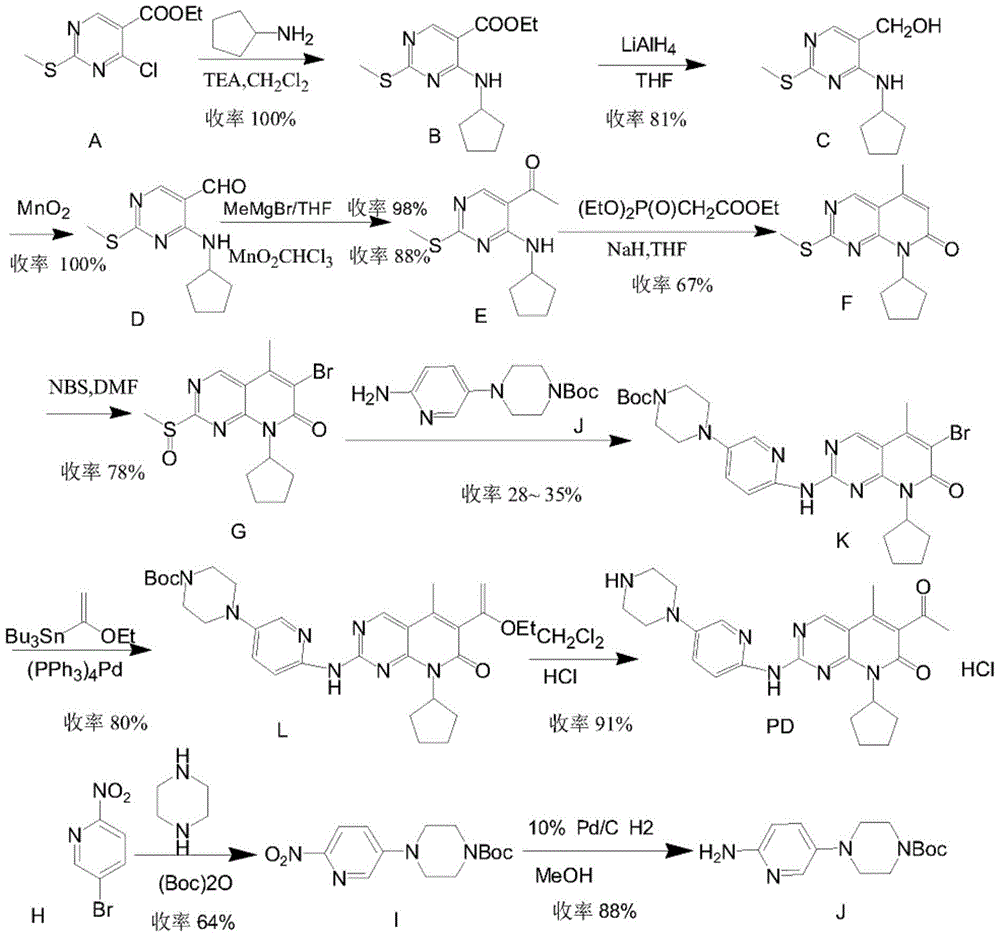
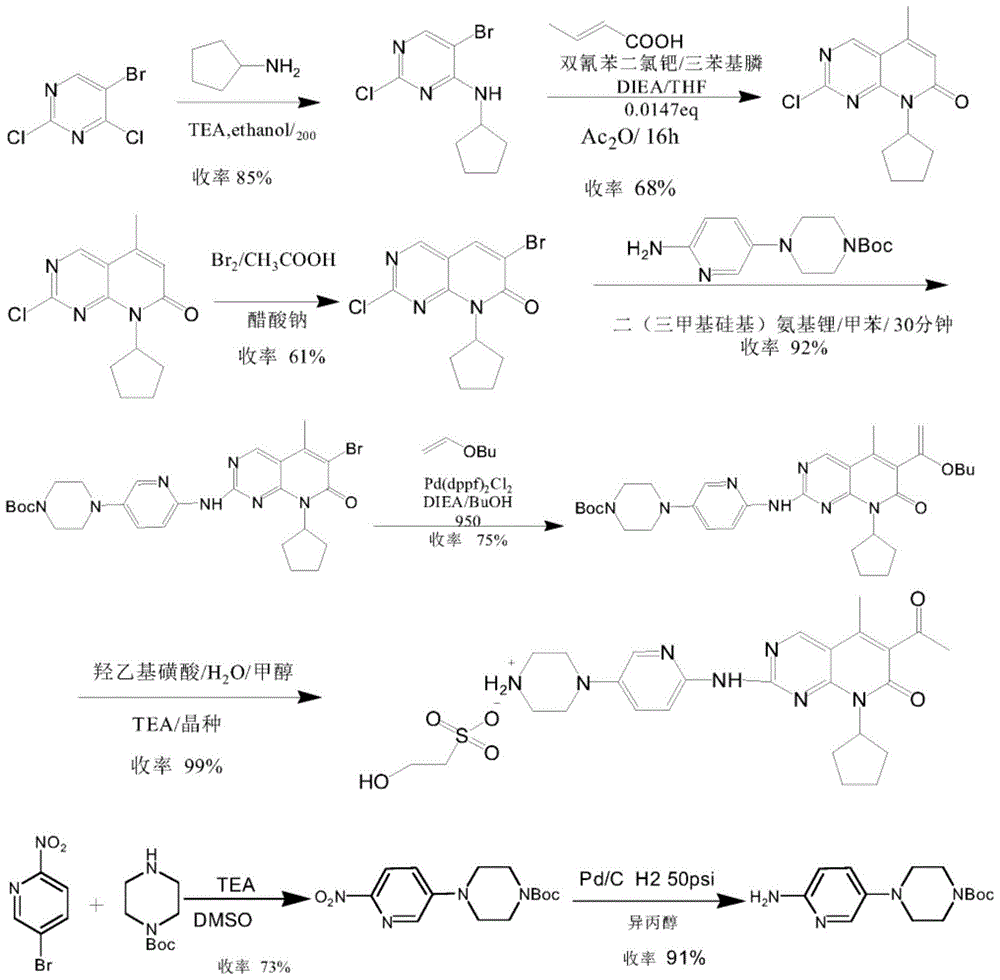
A kind of preparation method of palbociclib
A compound and reaction technology, applied in the field of preparation of palbociclib, can solve the problems of increasing the synthesis cost, etc., and achieve the effects of less waste, improved purity and yield, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the synthesis of compound IV
[0032] 1) Add 2-acetyl-2-butenoic acid methyl ester and N-[5-(1-piperazinyl)-2-piperidinyl]guanidine (compound III, 62.8g, 300mmol) and methanol 100mL In the CW-2000 ultrasonic-microwave reactor, add 16.4g of sodium methoxide dropwise at room temperature in 100mL of methanol solution, and stir for 15 minutes; add 11.9g of malononitrile and 2-acetyl-2-butenoic acid form Esters (21.3g, 150mmol) in 100mL of methanol solution, controlled microwave power: 100W, microwave frequency: 2450MHz, ultrasonic power: 50W, ultrasonic frequency: 20KHz, set the reaction temperature at 50°C, and the reaction time was 10min. TLC detected that the reaction was complete. A large amount of solids precipitated under ice bath, filtered with suction, washed the filter cake with a small amount of water, added ethanol and diethyl ether (1:1, V / V) for recrystallization, and dried in vacuo to obtain 57.0 g of compound IV with a yield of 95.8% and a purit...
Embodiment 2
[0034]Embodiment 2: the synthesis of compound V
[0035] 1) Add 21.2g (200mmol) of sodium dihydrogen hypophosphite, 100mL of water, 19.6g of concentrated sulfuric acid, and 39.6g (100mmol) of compound IV into the three-necked flask in sequence, and slowly add an aqueous solution of sodium nitrite (200mmol, 13.8 grams of sodium nitrite are dissolved in 10 milliliters of water), and the temperature of the control reaction system is lower than 0°C. After the dropwise addition was complete, stirring was continued for 4 hours. The reaction solution was extracted with ethyl acetate, and the ethyl acetate was recovered by rotary evaporation to obtain crude compound V; recrystallized from isopropanol to obtain 30.7 g of white crystal compound V with a product yield of 80.6% and a purity of 98.6% (HPLC).
[0036] 2) Add 31.8g (300mmol) of sodium dihydrogen hypophosphite, 100mL of water, 39.2g of concentrated sulfuric acid, and 39.6g (100mmol) of compound IV into the three-necked flask...
Embodiment 3
[0037] Embodiment 3: the synthesis of compound VI
[0038] 1) Add 38g (100mmol) of compound V, 11.5g of cyclopentane chloride, 14.2g of triethylamine, 0.072g of cuprous bromide, 0.099g of 1,10-phenanthroline and N,N-dimethyl 100 mL of methyl formamide, heated to 100°C, and stirred for 90 minutes. TLC detects that the reaction is complete. Add 200ml of water and stir evenly, solids are precipitated, suction filtered, and the obtained crude product is recrystallized with n-hexane and ethyl acetate (2:1, V / V), dried in vacuo to obtain 39.9 g of off-white solid compound VI; yield 88.7%, Purity 99.8% (HPLC method).
[0039] 2) Add 30.4g of compound V, 9.4g of chlorocyclopentane, 13.8g of anhydrous potassium carbonate, 0.057g of cuprous bromide, 0.079g of 1,10-phenanthroline and N,N-dimethyl 100 mL of methyl formamide was heated to 90°C, and the reaction was stirred for 120 minutes. TLC detects that the reaction is complete. Add 200ml of water and stir evenly, solid precipitate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com