Method for producing pentanediol through selective hydrogenolysis of furan derivative
A technology of furan derivatives and pentanediol, which is applied in the fields of chemical instruments and methods, chemical/physical processes, and the reduction and preparation of oxygen-containing functional groups. Uniform distribution, simple preparation method, good economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
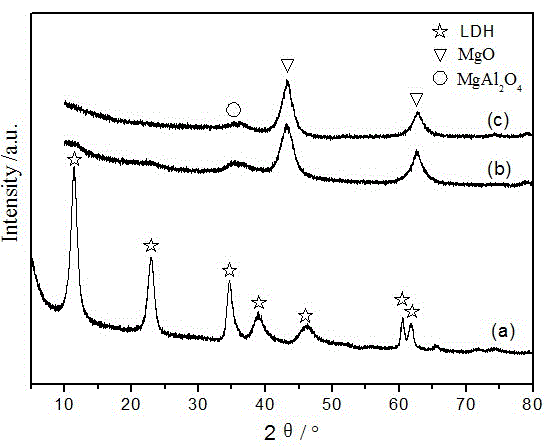
Image
Examples
Embodiment 1
[0028] A method for selective hydrogenolysis of furan derivatives to prepare pentanediol: 1) Preparation of active catalyst 1: Weigh 7.61g of copper nitrate trihydrate and 139.46g of aluminum nitrate tetrahydrate and dissolve them in 300mL of distilled water to form a salt solution, and weigh 40g Sodium hydroxide and 21.2g of anhydrous sodium carbonate were added to 200mL of water to make a mixed alkali solution, and the salt solution and mixed alkali solution were added dropwise to the three-necked flask under vigorous stirring to maintain the pH of the system at 10. Aged for 24 hours, suction filtered and washed with distilled water until neutral, and dried at 110°C for 12 hours to obtain a catalyst precursor with a layered structure of hydrotalcite. The precursor was calcined in a muffle furnace at 500° C. for 4 hours, and then reduced and activated in a hydrogen atmosphere at 350° C. for 3 hours to obtain the active catalyst 1 provided by the present invention.
[0029] 2)...
Embodiment 2
[0031] A method for selective hydrogenolysis of furan derivatives to prepare pentanediol: 1) Preparation of active catalyst 2: the operation is the same as in Example 1, replacing 139.46g of aluminum nitrate nonahydrate with 62.73g of zirconium nitrate tetrahydrate, and obtaining this product after reduction and activation Active catalyst 2 provided by the invention.
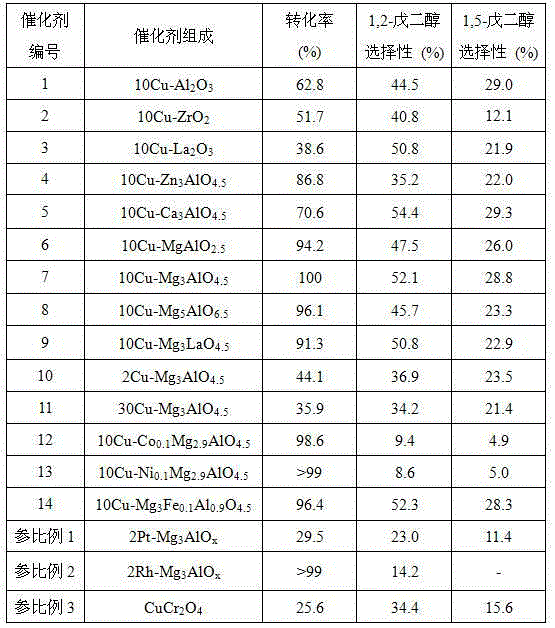
[0032] 2) Selective hydrogenolysis reaction of furfuryl alcohol: the same as in Example 1, and the test results are shown in Table 1.
Embodiment 3
[0034] A method for selective hydrogenolysis of furan derivatives to prepare pentanediol: 1) Preparation of active catalyst 3: the operation is the same as in Example 1, except that 47.84g of lanthanum nitrate hexahydrate is used instead of 139.46g of aluminum nitrate nonahydrate, and the product is obtained after reduction and activation The active catalyst 3 provided by the present invention.
[0035] 2) Selective hydrogenolysis reaction of furfuryl alcohol: the operation is the same as in Example 1, except that 40g of 10wt% furfuryl alcohol in methanol is replaced by 40g of 10wt% furfuryl alcohol in methanol solution. The test results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com