Method for synthesizing N-alkylthiophosphoryl triamide through continuous reaction
A technology of hydrocarbyl thiophosphoryl triamide and hydrocarbyl thiophosphoryl dichloride, which is applied in the field of continuous reaction synthesis of N-hydrocarbyl thiophosphoric triamide, which can solve post-processing difficulties, large production equipment, and cumbersome operation steps, etc. problems, to achieve the effects of improved production efficiency and safety, high production efficiency and safety, high mass transfer and heat transfer efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
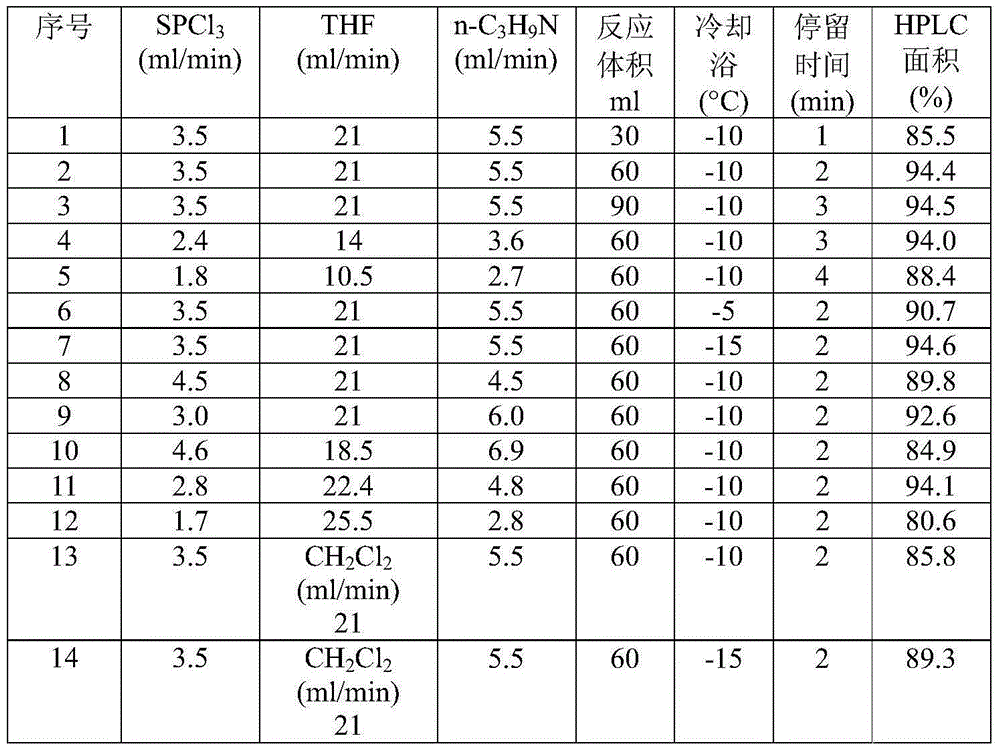
[0029] The preparation of embodiment 1N-n-propyl phosphorothioate dichloride
[0030] Phosphorus trichloride (SPCl 3 ), organic solvent (tetrahydrofuran THF or dichloromethane CH 2 Cl 2 ) and n-propylamine (n-C 3 h 9 N) Inject the micro-reactor system through the plunger pump respectively, the phosphorus trichloride and the organic solvent are firstly mixed through a mixer and then pre-cooled in the micro-heat exchanger (-5 ~ 0 ℃), and then mixed with the pre-cooled n-Propylamine (-5 ~ 0 ℃) was mixed in a cascaded mixer, and the obtained mixture entered a series of multi-stage micro-pipe reactors for delayed reaction. The inner diameter of each micro-pipe reactor was 200 μm and the volume was 30 mL. The micropipe reactor has an independent outlet. The temperature of the cooling bath in the reactor and the reaction residence time were controlled, and the intermediate reaction liquid was collected, and the contained N-n-propylphosphorothioate dichloride was characterized by...
Embodiment 2
[0033] The preparation of embodiment 2N-n-butyl thiophosphoryl dichloride
[0034] (1) Phosphorus trichloride (SPCl 3 ), organic solvent (tetrahydrofuran THF or dichloromethane CH 2 Cl 2 ) and n-butylamine (n-C 4 h 11 N) Inject the micro-reactor system through the plunger pump respectively, the phosphorus trichloride and the organic solvent are firstly mixed through a mixer and then pre-cooled in the micro-heat exchanger (-5 ~ 0 ℃), and then mixed with the pre-cooled n-Propylamine (-5 ~ 0 ℃) was mixed in a cascaded mixer, and the obtained mixture entered a series of multi-stage micro-pipe reactors for delayed reaction. The inner diameter of each micro-pipe reactor was 150 μm and the volume was 30 mL. The micropipe reactor has an independent outlet. The temperature of the reactor cooling bath and the reaction residence time were controlled, and the intermediate reaction liquid was collected, and the contained N-n-butylphosphorylthiodichloride was characterized by the HPLC ...
Embodiment 3
[0038] The preparation of embodiment 3N- isobutyl thiophosphoryl dichloride
[0039] Phosphorus trichloride (SPCl 3 ), organic solvent (tetrahydrofuran THF or dichloromethane CH 2 Cl 2 ) and isobutylamine (i-C 4 h 11 N) Inject the micro-reactor system through the plunger pump respectively, the phosphorus trichloride and the organic solvent are firstly mixed through a mixer and then pre-cooled in the micro-heat exchanger (-5 ~ 0 ℃), and then mixed with the pre-cooled n-Propylamine (-5 ~ 0 ℃) was mixed in a cascaded mixer, and the obtained mixture entered a series of multi-stage micro-pipe reactors for delayed reaction. The inner diameter of each micro-pipe reactor was 150 μm and the volume was 30 mL. The micropipe reactor has an independent outlet. The temperature of the reactor cooling bath and the reaction residence time were controlled, and the intermediate reaction liquid was collected, and the contained N-isobutylphosphorothioate dichloride was characterized by the HP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com