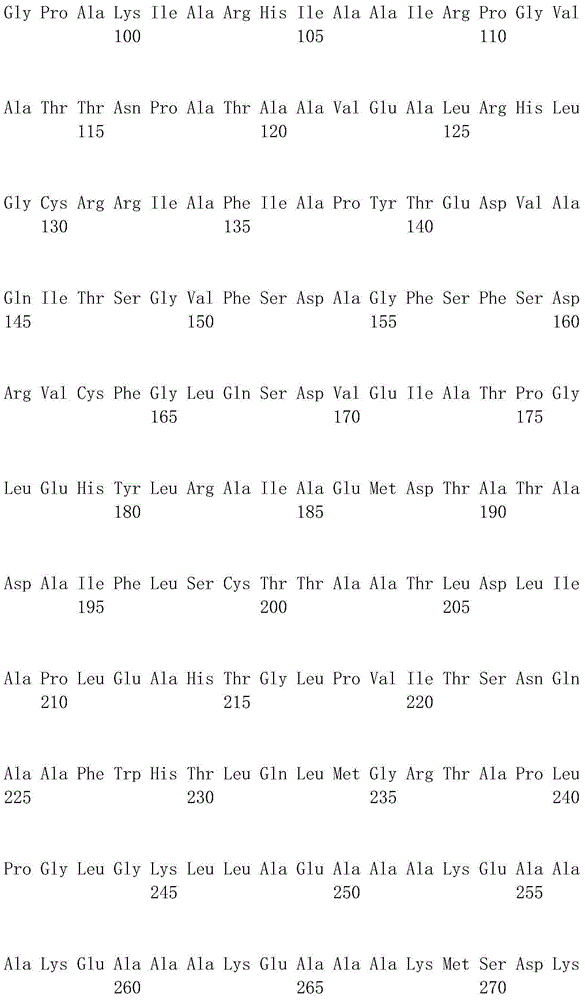Method for synthesizing L-cysteine through immobilized enzyme conversion of DL-ATC (DL-2-amino-delta<2>-thiazoline-4-carboxylic acid)
A cysteine and immobilized enzyme technology, applied in the field of bioengineering, can solve the problems of long substrate response time, insufficient enzyme amount, and stagnant progress, so as to improve operational stability, overcome easy leakage, and solve low activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1 Preparation of DL-ATC racemase / L-ATC hydrolase fusion protein
[0040] 1. Materials
[0041] Strains: Escherichia coli BL21 (DE3), purchased from Promega.
[0042] Plasmid: Plasmid pET28a(+) was purchased from Wuhan Miaoling Biotechnology Co., Ltd.
[0043] LB liquid medium: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L.
[0044] Kanamycin-resistant plate: LB solid medium containing 30 mg / L Kanamycin and 1.5% agar powder.
[0045] Kanamycin-resistant LB medium: LB liquid medium containing 30 mg / L kanamycin.
[0046] 2. Method
[0047] (1) Construction of atcAB expression vector
[0048] The DL-ATC racemase gene (atcA) (GenBanK accession number: BAD15357) and the L-ATC hydrolase gene (atcB) derived from the genome of Pseudomonas sp.BS strain genome (its sequence information exists in a section of GenBank accession A DNA sequence with a size of 10Kb (No. AB176845) was optimized for codons, and a sequence encoding a rigid linker peptide was added between ...
Embodiment 2
[0065] Embodiment 2 Preparation of recombinant nitrogen-carbamoyl-L-cysteine hydrolase
[0066] 1. Materials
[0067] Strains: Escherichia coli BL21 (DE3), purchased from Promega.
[0068] Plasmid: Plasmid pET28a(+) was purchased from Wuhan Miaoling Biotechnology Co., Ltd.
[0069] LB liquid medium is: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L.
[0070] Kanamycin-resistant plate: LB solid medium containing 30 mg / L Kanamycin and 1.5% agar powder.
[0071] Kanamycin-resistant LB medium: LB liquid medium containing 30 mg / L kanamycin.
[0072] 2. Method
[0073] (1) construction of atcC expression vector;
[0074]The nitrogen-carbamoyl-L-cysteine hydrolase gene (atcC) (its sequence information exists in a section of GenBanK accession number AB176845 that is derived from the nitrogen-carbamoyl-L-cysteine hydrolase gene (atcC) of Pseudomonas sp.BS strain genome is 10Kb in size DNA sequence) for codon optimization, the optimized gene sequence is shown in SEQ ID NO.5, t...
Embodiment 3
[0091] Example 3 Construction of co-immobilized multienzyme system
[0092] The recombinant fusion protein AtcAB50mg obtained in Example 1 and the recombinant protein AtcC25mg obtained in Example 2 were added to 12.5mL of 80g / L polyvinyl alcohol and 15g / L sodium alginate at the same time and adjusted to pH 7.5 with 0.1N NaOH. In the aqueous solution, until the final concentration of total protein is 6 mg / mL, after mixing well, add 750 μL glutaraldehyde solution with a mass fraction of 10%, mix well, and then conduct cross-linking reaction at 4°C for 3 hours. The reaction solution was dripped dropwise into 20 g / L calcium chloride solution with a No. 6 needle syringe, and the small spheres were filtered out. Place the small spheres in 20g / L calcium chloride solution, soak and harden for 1 hour. After filtering out the small spheres, cross-link with 400 mL of 0.02% glutaraldehyde solution at 4° C. for another 3 h, and then filter out the small spheres. With pH 7.5 containing 15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com