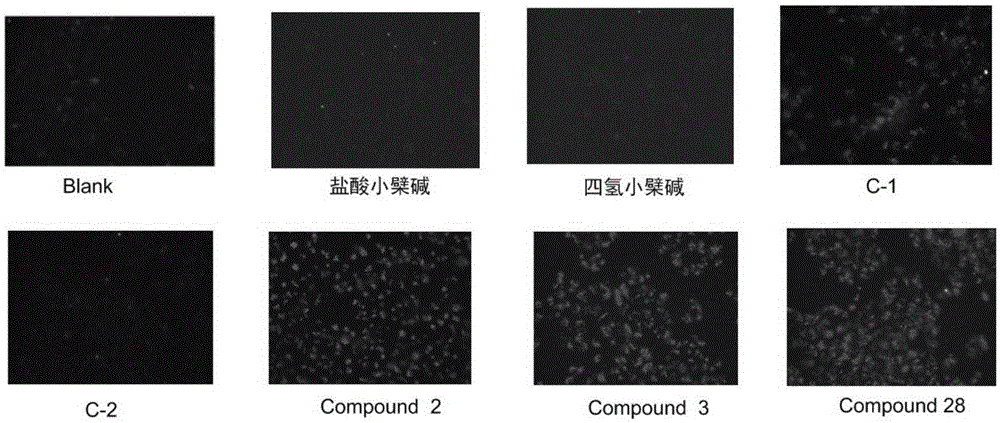
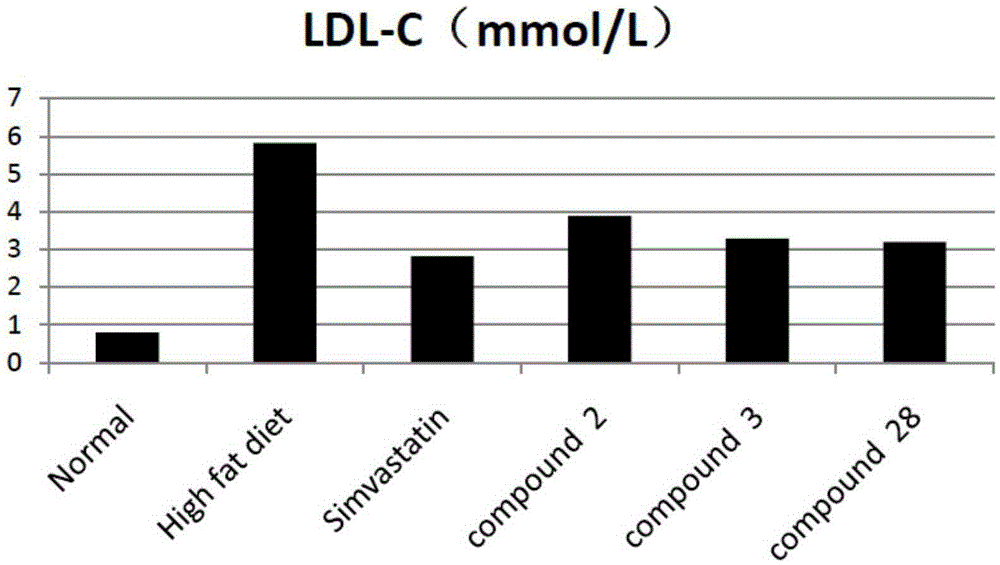
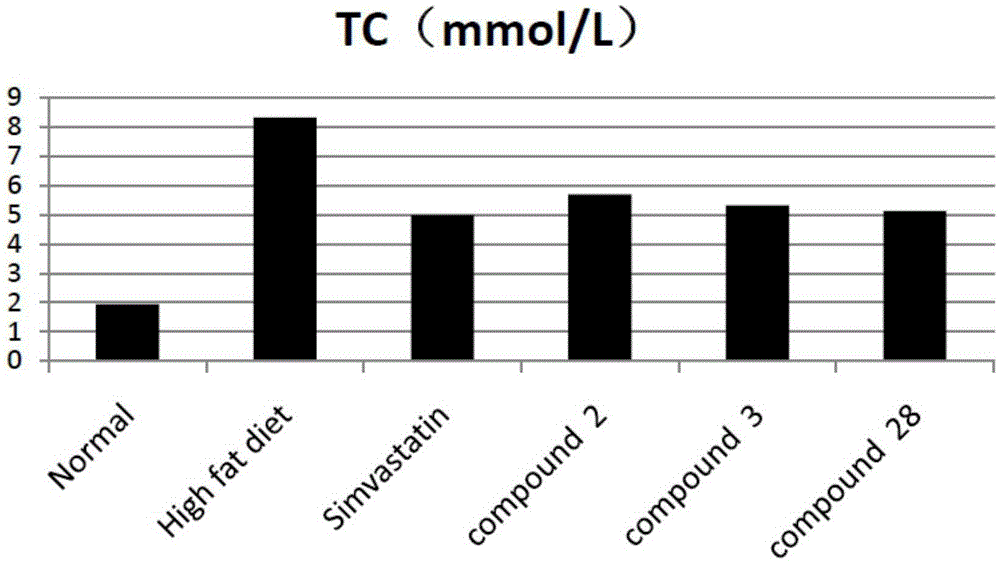
Tetrahydrocoptisine derivative and applications thereof
The technology of a compound and a medicinal salt, which is applied to tetrahydrocoptisine derivatives and their application fields, can solve problems such as visual impairment, gallstones, and insignificant hypolipidemic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0237] The general method of the present invention is further explained below, the compound of the present invention can be prepared by methods known in the art, and the preparation method of the preferred compound of the present invention is used as an example to illustrate in detail below, but the preparation method of the compound of the present invention is not limited thereto .
[0238] The preparation of the general formula compound (VII) disclosed in the present invention is mainly prepared according to the following scheme:
[0239]
[0240] The above preparation route is described as follows: V1 and V2 are the starting materials of this scheme, which can be obtained from commercially available products, or prepared according to methods reported in literature. First, V1 and V2 are dehydrated under a certain reaction temperature to obtain an imine compound V3, and the compound V3 is reduced to an "amine" compound V4. Under certain reaction conditions, V4 reacts with ...
specific Embodiment
[0242]Representative process: Now take the oxo group substitution at the 4th position of the D ring (such as acetoxy, benzoyloxy, benzyloxy, sulfonyloxy, phosphoryloxy, etc.) as an example, and give a brief description:
[0243]
[0244] Basic process: First, react substituted phenylethylamine S6 and substituted o-hydroxybenzaldehyde S17 in dichloromethane at 60°C to generate imine compound S18, concentrate dichloromethane under reduced pressure, add methanol solution, Reduction reaction under a certain temperature condition, remove the methanol solvent under reduced pressure, disperse with ethyl acetate, wash with saturated brine for 3 times, combine the ethyl acetate layer, concentrate under reduced pressure to obtain the "amine" compound S19, and S19 with formic acid Disperse, add copper sulfate and glyoxal, reflux reaction at 80°C, then keep warm for 2 hours, a large amount of solids will precipitate, filter the solids, disperse with methanol, add calcium oxide to adjust...
Embodiment approach
[0258]
[0259] Basic process: first, the natural product Z1 is demethylated by vigorous stirring reaction at a high temperature of 200°C to obtain compound Z2, and then prepared by reacting with sulfuryl chloride in a dichloromethane solvent at a low temperature of -10-5°C to room temperature Compound Z3 was reduced by sodium borohydride in methanol solution at low temperature to obtain compound Z4.
[0260] The present invention will be further described below through specific examples, but those skilled in the art should know that the present invention is not limited to these examples.
[0261] Compound structures were determined by nuclear magnetic resonance (NMR) or mass spectroscopy (MS). The mensuration of NMR is to use BrukerAVANCE-400 nuclear magnetic instrument, and measuring solvent is deuterated dimethyl sulfoxide (DMSO-d 6 ), deuterated chloroform (CDCl 3 ), deuterated methanol (CD 3 OD), the internal standard is tetramethylsilane (TMS), and the chemical shi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com