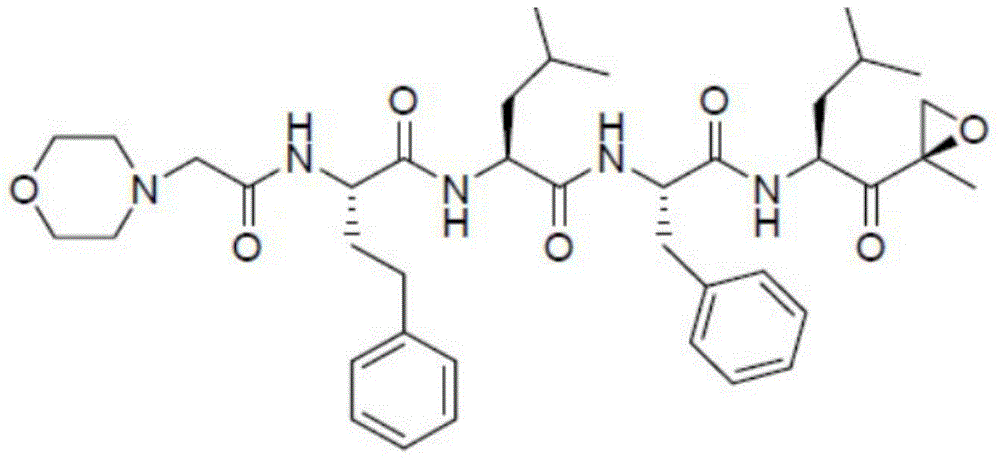
Liposome lyophilized composition of carfilzomib drug and preparation method thereof
A technology of liposome freeze-drying and carfilzomib, which can be used in drug combinations, medical preparations of inactive ingredients, and freeze-dried delivery, etc., and can solve problems such as phlebitis, vasospasm, and hidden dangers.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
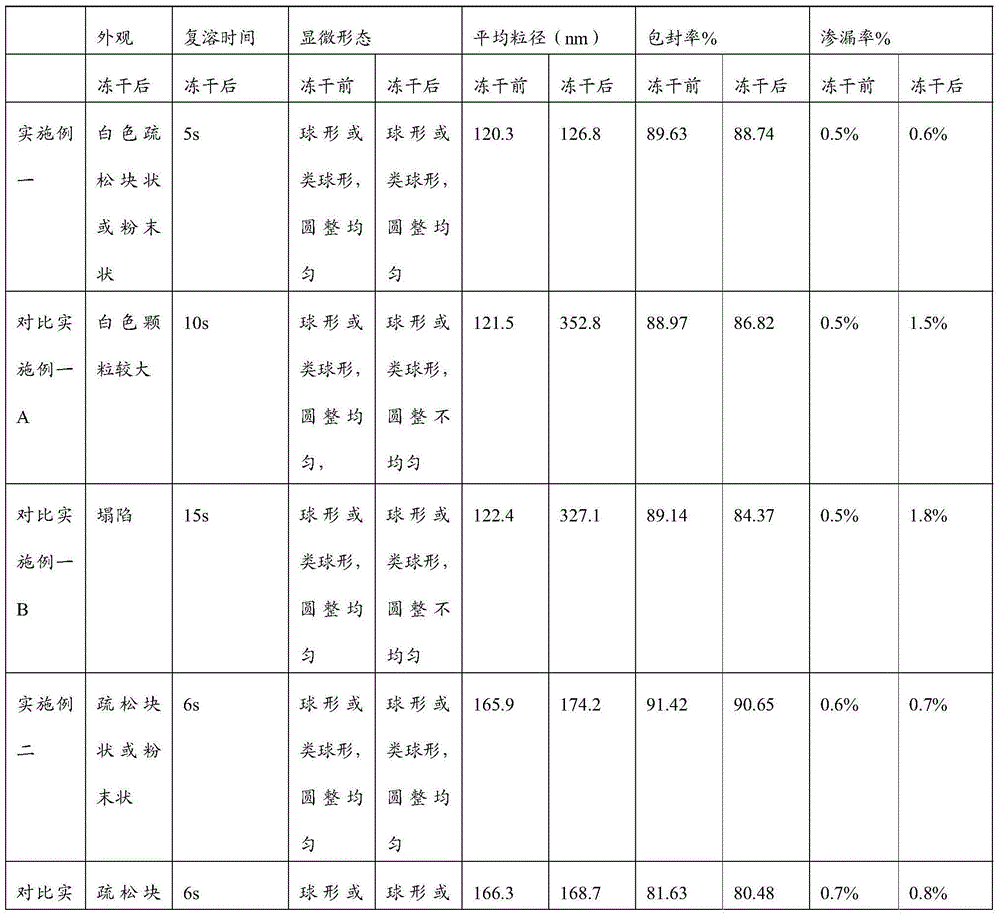
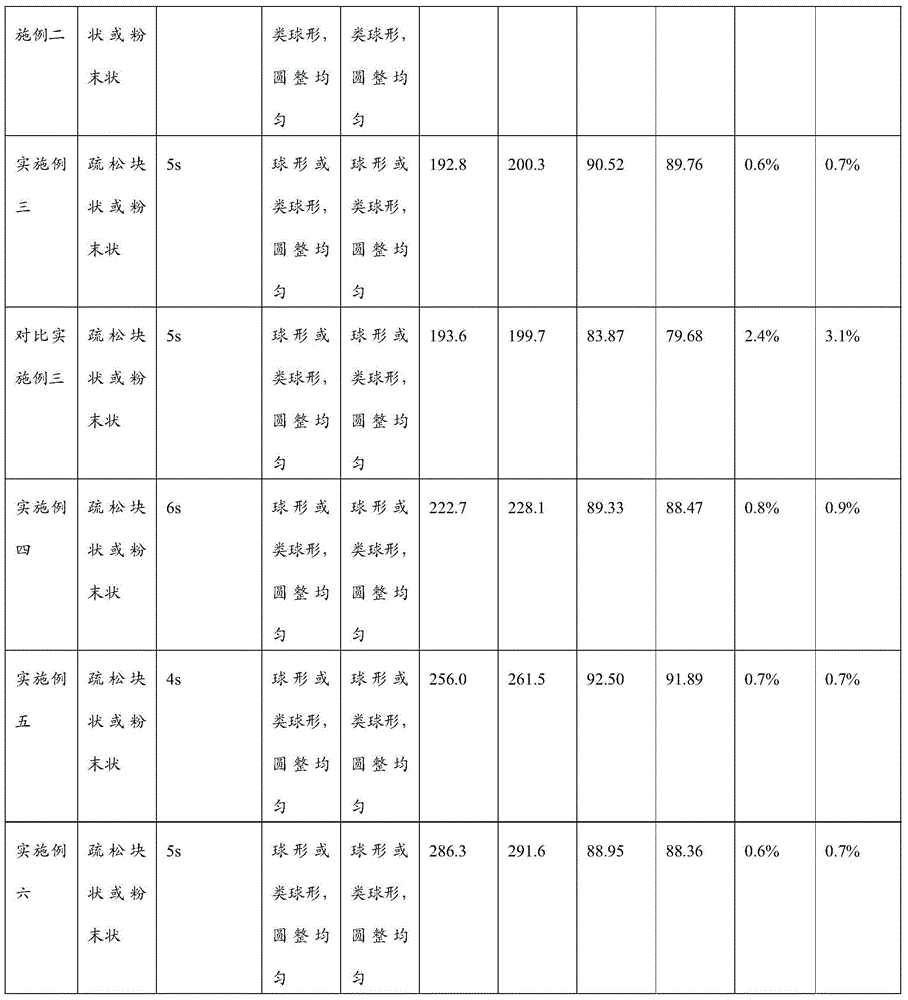
Image
Examples
Embodiment 1
[0045] Embodiment one (medicine 30-100mg) 1000 dosage units
[0046] 1. Prescription
[0047] Carfilzomib
60g
Vitamin E
2.25g
15g
High Purity Hydrogenated Egg Yolk Lecithin
375g
60g
45g
45g
5000ml
[0048] 2. Preparation process
[0049] Weigh high-purity hydrogenated egg yolk lecithin and cholesterol in a pear-shaped bottle, add an appropriate amount of chloroform or anhydrous ether to dissolve completely, add carfilzomib raw material powder to dissolve completely, and use a rotary evaporator at a temperature of 40°C to rotate and evaporate under reduced pressure Remove the organic solvent, after forming a uniform film on the inner wall of the bottle, blow it dry with nitrogen or continue to rotate under negative pressure for 10 minutes to ensure that the organic solvent is removed, add a phosphate buffer soluti...
Embodiment 2
[0069] 1. Prescription
[0070] Carfilzomib
50g
Vitamin E
2g
15g
Distearoylphosphatidylcholine (DSPC)
275g
cholesterol
50g
35g
40g
5000ml
[0071] 2. Preparation process
[0072] Weigh distearoylphosphatidylcholine (DSPC) and cholesterol in a pear-shaped bottle, add an appropriate amount of chloroform or anhydrous ether to dissolve completely, add carfilzomib raw material powder to dissolve completely, and use rotary evaporation at a temperature of 38°C Remove the organic solvent by rotary evaporation under reduced pressure in the bottle. After forming a uniform film on the inner wall of the bottle, blow it dry with nitrogen or continue the negative pressure rotary evaporation for 10 minutes to ensure that the organic solvent is removed. Add a phosphate buffer solution containing vitamin E ( pH 6.8), shake well, let stand for...
Embodiment 3
[0084] 1. Prescription
[0085] Carfilzomib
70g
Vitamin C
5g
citric acid
40g
High Purity Hydrogenated Soy Lecithin (HSPC)
600g
cholesterol
150g
100g
100g
2500ml
[0086] 2. Preparation process
[0087] Weigh high-purity hydrogenated soybean lecithin (HSPC) and cholesterol in a pear-shaped bottle, add an appropriate amount of chloroform or anhydrous ether to dissolve completely, add carfilzomib raw material powder to dissolve completely, and reduce it with a rotary evaporator at a temperature of 45°C. Remove the organic solvent by pressure rotary evaporation. After a uniform film is formed on the inner wall of the bottle, blow it dry with nitrogen or continue negative pressure rotary evaporation for 10 minutes to ensure that the organic solvent is completely removed. Add a phosphate buffer solution (pH6. 8), shake well, let stand for 20 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com