Thienopyrroloquinone compound, a preparation method and application thereof as a semiconductor active layer in an organic field effect transistor
A compound and thiophene technology, applied in the field of thienopyrrole quinone compound, preparation method and semiconductor equipment containing the material, can solve the problems of limited quantity, low bipolar field-effect mobility, etc., and achieve strong self-assembly ability , Low LUMO energy level, narrow energy level bandgap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
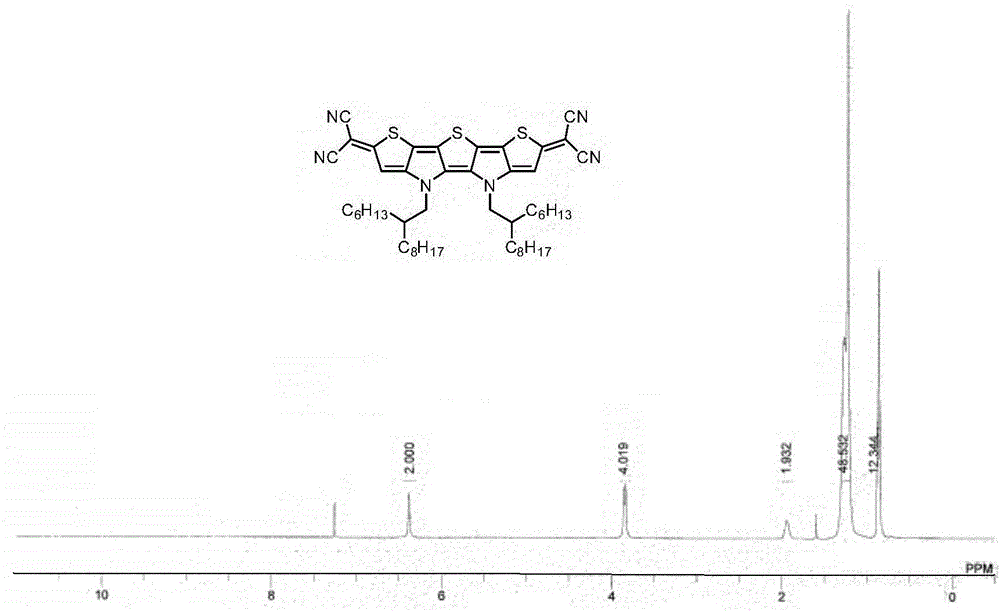
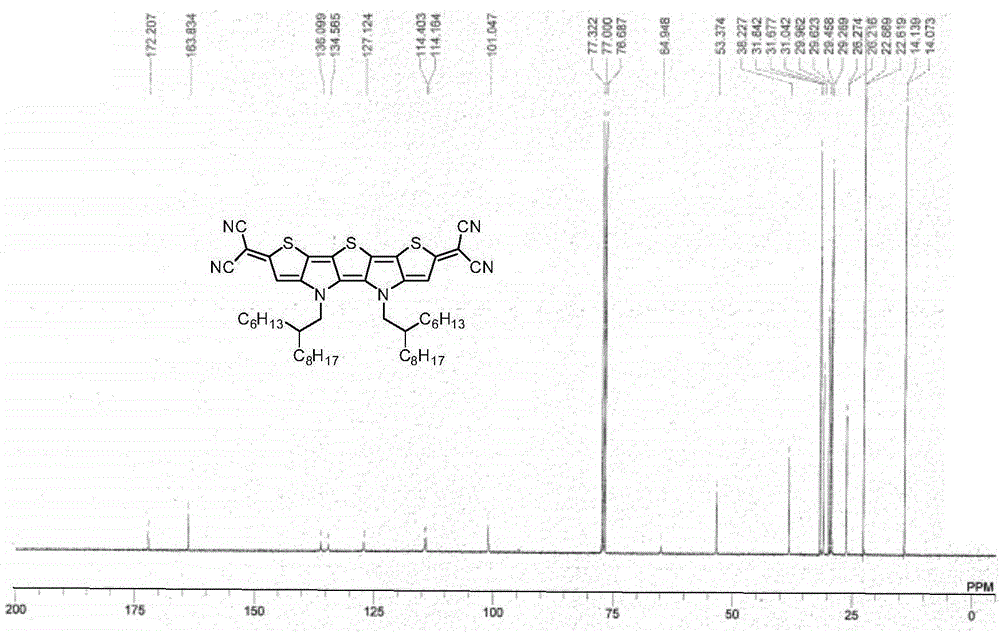
[0037] Example 1: Synthesis of Compound Ia
[0038]
[0039] To a 20 mL three-necked flask containing sodium hydride (56.0 mg, 60 wt%, 1.4 mmol) and 1,2-dimethoxyethane (5 mL) at 0°C under nitrogen protection, malononitrile (46.2 mL) was added in one portion. mg, 0.7 mmol), the foam was removed and the reaction was raised to room temperature for 30 minutes. The prepared malononitrile anion solution was transferred via cannula to a solution containing compound IIa (136.5 mg, 0.14 mmol), tetrakis(triphenylphosphine)palladium (32.4 mg, 0.028 mmol) and 1,2-dimethoxy In a 50 mL three-necked flask of ethyl ethane (10 mL), the reaction was heated and refluxed for 3 hours under nitrogen protection. Then the reaction temperature was lowered to room temperature and exposed to air, diluted hydrochloric acid (10 mL, 1 M) was added, stirred in an ice-water bath for 30 minutes, extracted with ether (30 mL×3), the organic phases were combined and washed with saturated brine, After dryin...
Embodiment 2
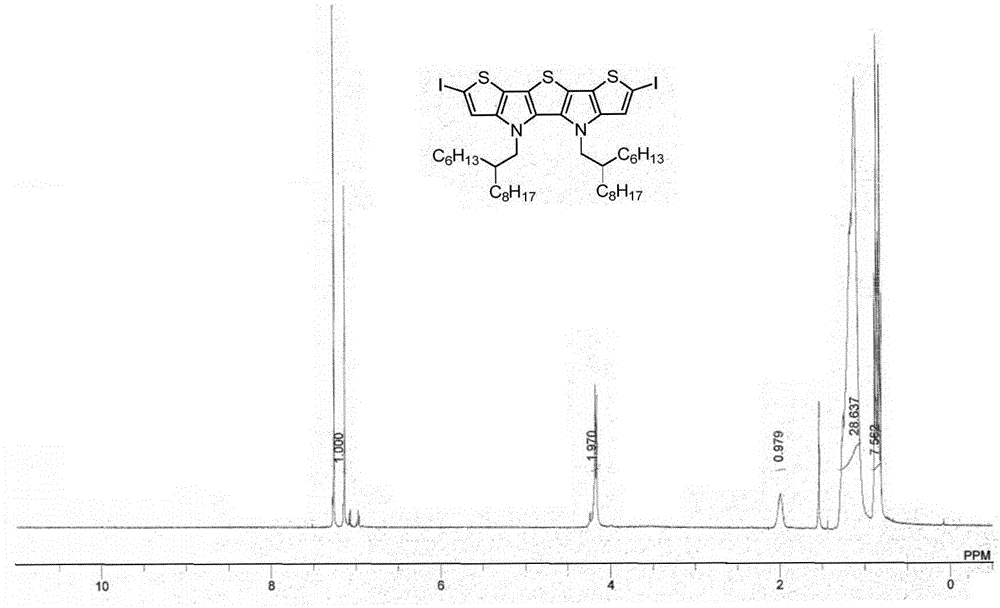
[0040] Example 2: Synthesis of Compound IIa
[0041]
[0042] To a 10 mL three-necked flask containing compound IV (130.2 mg, 0.18 mmol) and tetrahydrofuran (2 mL) at -78 °C under nitrogen protection, n-butyllithium (1.6 M in hexane, 248 μL, 0.396 mmol) was slowly added dropwise, Keep stirring at low temperature for 30 minutes, add elemental iodine (100.5mg, 0.396mmol), warm to room temperature, continue stirring for 2 hours, add saturated sodium thiosulfate solution (10mL) for quenching, extract with ether (30mL×3), The organic phases were combined, washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was removed by rotary evaporation to obtain the crude product Compound IIa (yellow oil, 173.8 mg, yield: 99%). 1 H NMR (400MHz, CDCl 3 )δ7.14(s,2H),4.17(d,J=8.0Hz,4H),1.99(m,2H),1.26–1.12(m,48H),0.89–0.80(m,12H); 13 C NMR (100MHz, CDCl 3 )δ144.0,129.9,121.2,120.8,116.3,68.8,53.5,39.0,31.9,31.7,31.0,29.9,29.6,29.4,29.3,26.13,26.09,22.7,22.6,1...
Embodiment 3
[0043] Example 3: Synthesis of Compound IV
[0044]
[0045] Compound V (282.0 mg, 0.5 mmol), sodium tert-butoxide (768.8 mg, 8.0 mmol), bis(dibenzylideneacetone) palladium (28.8 mg, 0.05 mmol), 1,1' were added to a 50 mL three-necked flask. -Bis(diphenylphosphino)ferrocene (110.9 mg, 0.2 mmol) and toluene (10 mL), stirred at 25°C for 20 minutes, added compound VI (280.1 mg, 1.16 mmol), heated to 110°C and reacted for 10 hours . After cooling to room temperature, water (20 mL) was added, extracted with ether (30 mL×3), the organic phases were combined and washed with saturated brine, dried over anhydrous magnesium sulfate, and the organic solvent was removed by rotary evaporation. The residue was separated by silica gel column chromatography ( Eluent: n-hexane) to obtain compound IV (white solid, 181.0 mg, yield: 50%). 1 H NMR (400MHz, CDCl 3 )δ7.06(d,J=4.8Hz,2H),6.98(d,J=5.6Hz,2H),4.26(d,J=8.0Hz,4H),2.06(m,2H),1.25–1.11( m,48H),0.89–0.81(m,12H); 13 C NMR (100MHz, CDCl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com