Melanin precursor substance hair dye and using method thereof
A technology of melanin precursor and hair dye, which is applied in fermentation, hair care, pharmaceutical formulation and other directions, can solve the problems of unstable performance, high cost, long time consumption, etc., and achieves safe and non-toxic use effect, reliable use effect and safety. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] The preparation method of the present embodiment melanin precursor is carried out according to the following steps:
[0078] 1. Extraction and determination of tyrosinase
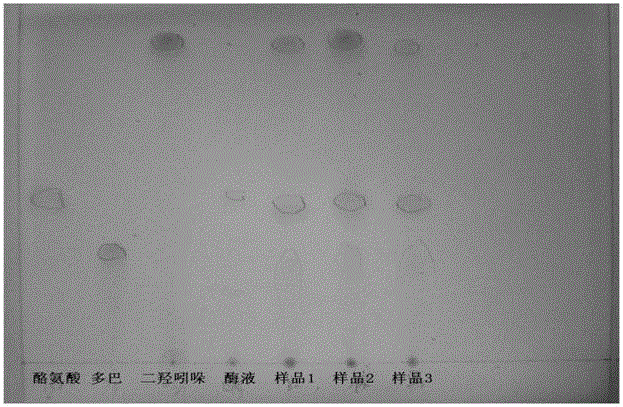
[0079] Take 500 grams of crushed Pleurotus eryngii and add 1000 ml of distilled water to extract the tyrosinase solution or use 1000 ml of neutral phosphate buffer to extract the tyrosinase solution.
[0080] The measurement method of tyrosinase activity is as follows: Take 0.1ml of mushroom compound enzyme solution and 3.9ml of substrate solution to react at 317nm for 10min, and the enzyme activity unit U is defined as 0.01 units of absorbance change per 1min is 1 enzyme activity unit. When the measured result is greater than 1000U, the enzyme solution can be used for the following melanin precursor accumulation reaction.
[0081] 2. Accumulation reaction of melanin precursor
[0082] Prepare 2L of tyrosine solution with a concentration of 1mM-10mM, add the tyrosine substrate solution into a bior...
Embodiment 2
[0089] The preparation method of the present embodiment melanin precursor is carried out according to the following steps:
[0090] 1. Extraction of Tyrosinase
[0091] Use a juice extractor to crush the mushrooms, add 500g fruiting body residue to 1000ml, extract the enzyme solution with a citric acid buffer solution with a pH value of 6.8, use 5% activated carbon to absorb and treat, and then centrifuge for later use.
[0092] 2. Immobilization of Tyrosinase
[0093] 200-mesh diatomite was heat-treated at 60°C for 30 minutes, soaked in phosphate buffer for 20 minutes, centrifuged to remove the supernatant, and the treated diatomite was added to the above enzyme solution for 5 hours to obtain immobilized enzyme for use .
[0094] 3. Accumulation reaction of melanin precursors
[0095] Prepare 2L of tyrosine solution with a concentration of 5mM, which can be completely dissolved by heating and dissolving.
[0096] Add the tyrosine substrate solution into a bioreactor with ...
Embodiment 3
[0103] The preparation method of the present embodiment melanin precursor is carried out according to the following steps:
[0104] 1. Extraction of Tyrosinase
[0105] Use a juice extractor to crush Agaricus bisporus, add 500g of crushed residue to 1000ml of Tris-hydrochloric acid buffer solution with a pH value of 7.0 to extract the enzyme solution, centrifuge to extract the supernatant, and use 10% macroporous adsorption resin to adsorb polysaccharides and miscellaneous proteins in the enzyme solution. The treated enzyme solution was stored at 4°C for later use.
[0106] 2. Accumulation reaction of melanin precursor
[0107] Prepare 2L of tyrosine solution with a concentration of 10mM, which can be completely dissolved by heating and dissolving.
[0108] Reaction process is with embodiment 1.
[0109] The liquid sample taken out was filtered through filter paper, and after the filtrate was diluted 10 times, the absorbance at 300 nm and 475 nm was measured in an ultraviol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com