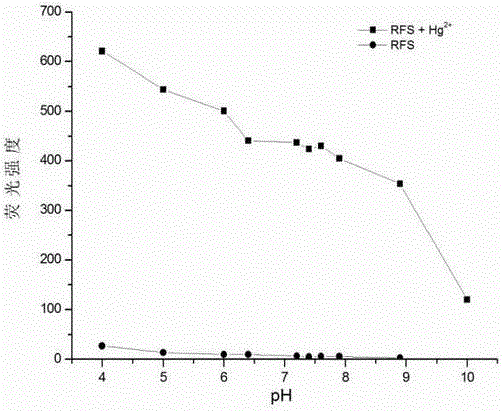
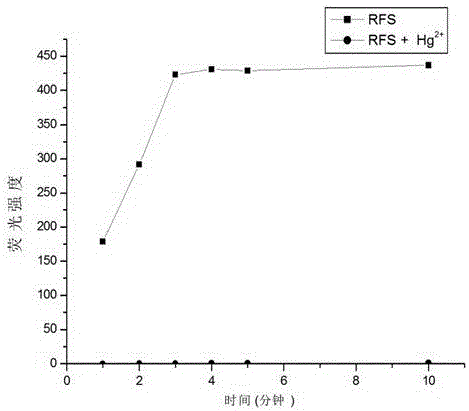
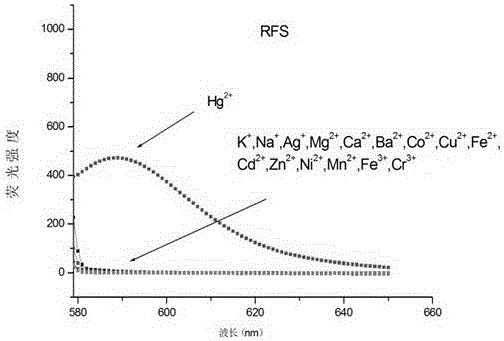
Mercury ion fluorescent probe and preparation method and application thereof
A fluorescent probe, mercury ion technology, applied in the field of fluorescent probes, can solve the problems of high equipment requirements, high price, and no continuous detection, and achieve the effects of simple synthesis steps, high specificity, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033] Synthesis of rhodamine B hydrazide compound
[0034]Take 1.443 g (3 mmol) of Rhodamine B and transfer it into a 100 mL round bottom flask, dissolve it with 50 mL of ethanol, then pipette 3 mL (80 wt%, 20 mmol) of hydrazine hydrate aqueous solution with a pipette gun, and the reaction system is now Deep wine red, reflux reaction in an oil bath at 85°C, monitor the progress of the reaction by thin-layer chromatography (TLC), the developer is a mixed solution of petroleum ether and ethyl acetate with a volume ratio of 1:1, react for 6 hours and spot the plate It was found that the raw material points basically disappeared on the thin-layer plate, and the rhodamine hydrazide product point appeared at the position of about 0.5 in the ratio shift value. After slight heating, the product point was rose red. The reaction was quenched, the system was extracted with ethyl acetate, and the organic phase was collected and then distilled under reduced pressure to obtain ...
Embodiment 2
[0036]
[0037] Synthesis of o-fluororhodamine probe RFS
[0038] Take 0.1238 g (0.75 mmol) of 2-fluorophenylisothiocyanate and 0.2292 g (0.5 mmol) of rhodamine hydrazide compound and transfer them to a 25 mL round bottom flask, then add 5 mL of dry isopropanol, Magnetically stir the reflux reaction in an oil bath at 85°C, and monitor the reaction by TLC after reacting for 5 minutes. The developer is a mixed solution of petroleum ether and ethyl acetate with a volume ratio of 1:1. After 10 hours, the raw material point disappears, and the reaction is stopped. , after the system was lowered to room temperature, the solvent was distilled off under reduced pressure and neutral alumina was added to its dry-mixed sample, and neutral alumina was used as the stationary phase for column chromatography (petroleum ether: ethyl acetate=10:2, v / v) Separation and purification, the product is white fluffy powder ortho-fluororhodamine probe RFS (yield 82%).
Embodiment 3
[0040] Synthesis of m-fluororhodamine probe RFSM
[0041] Take 0.1238 g (0.75 mmol) of 3-fluorophenylisothiocyanate and 0.2292 g (0.5 mmol) of rhodamine hydrazide compound and transfer them to a 25 mL round bottom flask, then add 5 mL of dry isopropanol, Magnetically stir the reflux reaction in an oil bath at 85°C, and monitor the reaction by TLC after reacting for 5 minutes. The developer is a mixed solution of petroleum ether and ethyl acetate with a volume ratio of 1:1. After 10 hours, the raw material point disappears, and the reaction is stopped. , after the system was lowered to room temperature, the solvent was distilled off under reduced pressure and neutral alumina was added to its dry-mixed sample, and neutral alumina was used as the stationary phase for column chromatography (petroleum ether: ethyl acetate=10:2, v / v) Separation and purification, the product is white fluffy powder m-fluororhodamine probe RFSM (yield 74%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com