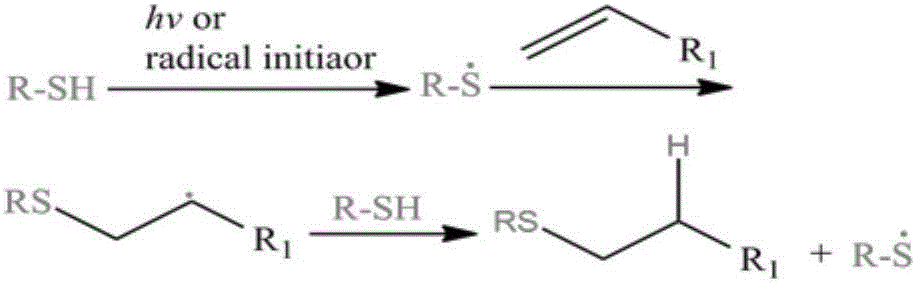
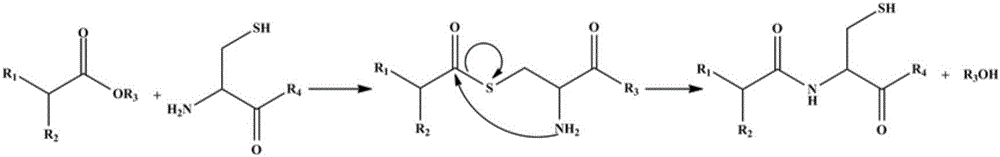
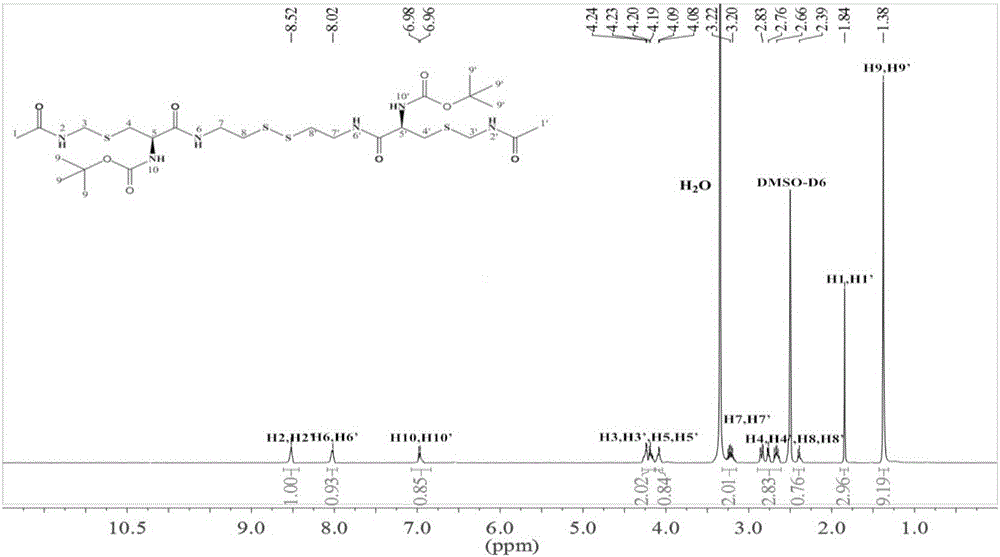
Cysteine-hyaluronic acid conjugate prepared through freeze drying and thiol-ene click chemistry, synthesis method and applications thereof
A technology of hyaluronic acid and cysteine, applied in the field of biomedicine, can solve the problems of CD44 receptor not being able to recognize normally, reducing cell repair damage, unable to achieve in situ injection, etc., achieving broad biomedical prospects and increasing cell adhesion. good rheological properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0101] The preparation method of the cysteine-functionalized hyaluronic acid conjugate prepared by freeze-drying and thiol-ene click chemistry comprises the following steps:
[0102] 1) Take the structural formula as The compound 1 hyaluronic acid with the structural formula is The compound 2 formed by freeze-drying method has the structural formula: Compound 3;
[0103] 2) Take the structural formula as The compound 4 and the structural formula are The compound 5 forms the structural formula as Compound 6;
[0104] 3) Compound 6 undergoes a reduction reaction with a disulfide bond reducing agent to form a structural formula of Compound 7 of
[0105] 4) Taking compound 3 and compound 7, under photoinitiator and ultraviolet light irradiation, thiol-ene click chemistry forms the structural formula as Compound 8;
[0106] 5) Remove the tert-butoxycarbonyl and acetamidomethyl protecting groups on compound 8 respectively to obtain the structural formula: Compound...
Embodiment 1
[0120] 1) Synthesis of compound 3
[0121]
[0122] Synthetic scheme of compound 3 in table 1
[0123]
[0124]Remarks: 1g 100,000 hyaluronic acid contains 2.636mmol primary alcohol hydroxyl groups; allyl glycidyl ether molecular weight: 114.14g / mol density: 0.962g / ml boiling point: 154°C (lit.)
[0125] Take 14g of hyaluronic acid (100kDa), dissolve it in 700ml of ultrapure water in a 1000ml round bottom flask to prepare a hyaluronic acid aqueous solution with a concentration of 2% (w / v), and prepare seven 250ml round bottom flasks numbered 1 to 7 Flasks, fill each flask with 100ml of 2% hyaluronic acid solution for later use, take 9.76ml of allyl glycidyl ether, add ultrapure water to 100ml with a volumetric flask and mix evenly, add the The corresponding volume of allyl glycidyl ether aqueous solution was stirred and mixed evenly at room temperature, transferred to a petri dish, frozen at -80°C, and freeze-dried to obtain a series of modified allyl glycidyl ether hya...
Embodiment 2
[0154] The specific route of the covalently cross-linked hyaluronic acid hydrogel that can be formed in situ by injection mediated by the natural chemical ligation reaction is as follows:
[0155]
[0156] Experimental steps:
[0157] Solution A: Weigh 35 mg of the cysteine-hyaluronic acid conjugate (Cys-HA) with a series of modification ratios synthesized in Example 1 and dissolve it in a 1 ml centrifuge tube with 350 ul of phosphate buffer (pH 7.6).
[0158] Solution B: Weigh 35mg of 10k four-branch polyethylene glycol oxyester conjugate (4-ARM-PEG-S-S) and dissolve it in 350ul of phosphate buffer (pH7.6) in a 1ml centrifuge tube.
[0159] Solution A and solution B were quickly mixed with a vortex mixer and added to the rheometer for testing. Time sweep is performed first, frequency sweep is performed when the modulus curve no longer changes, and finally strain sweep is performed. The parameter settings are as follows.
[0160] The parameters in the rheological test are...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com