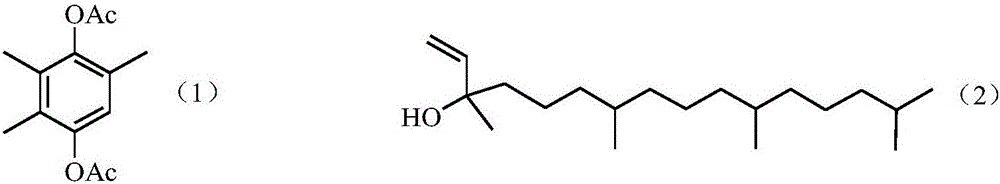
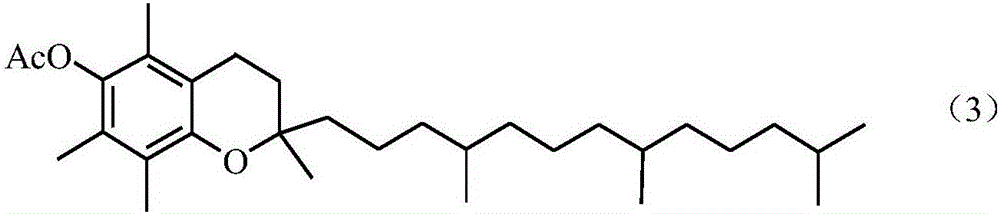
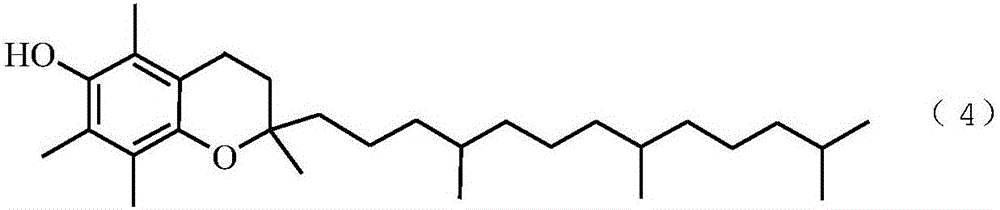
Method of preparing vitamin E acetate
A technology for acetate and vitamin, applied in the field of preparation of vitamin E acetate, can solve problems such as difficulty in large-scale industrial production, difficult treatment of process waste liquid, corrosion of catalyst to equipment, etc., and achieves reactivity and product selectivity. The effect of improving, improving product quality, and stabilizing product vitamin E acetate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Cocatalyst 1 preparation method is as follows:
[0058] Preparation of MCM-48 molecular sieve: Add 10g of cetyltrimethylammonium bromide (CTAB) into 100g of pure water, then add 110g of methanol and 20g of concentrated ammonia water respectively, after ultrasonication at room temperature for 30min, add 15g of ethyl orthosilicate dropwise Ester (TEOS), ultrasonicated for 30 minutes at room temperature, filtered with suction and washed with pure water to pH = 7, dried at 120°C for 2 hours and ground into powder, then placed in a muffle furnace and roasted at 800°C for 4 hours to obtain MCM- 48 molecular sieves.
[0059] A 1 Xa / A 2 / MCM-48 molecular sieve preparation: under nitrogen protection conditions, 2.5g FeCl 2 Add 1.0g of nanoscale Fe particles into 400g of pure water, stir and mix fully at room temperature, add 60g of the prepared MCM-48 molecular sieve into it, stir fully at 50°C for 2h, and dry at 120°C for 6h after stirring to obtain FeCl 2 / Fe / MCM-48 molecu...
Embodiment 2-7
[0061] The preparation method of cocatalyst 2-7 is similar to that of cocatalyst 1, and the material ratio and detailed material composition are shown in Table 1 below:
[0062] Table 1 cocatalyst 2-7 preparation raw material formula table
[0063]
[0064]
Embodiment 8
[0066] Add 70.0g of TMHQ-DA, 30.2g of acetic anhydride and 142.3g of glacial acetic acid into a 500mL three-neck flask, heat to 70°C to completely dissolve TMHQ-DA, start stirring, add 1.40g of cocatalyst 1, and pass through at a speed of 7.4mL / min Add hydrogen chloride gas, then start to add IPL (87.9g) dropwise, add dropwise for 120min, continue to react for 60min after the dropwise addition is completed, stop feeding hydrogen chloride gas, sample analysis, the conversion rate of raw material TMHQ-DA is 99.6%, and the product vitamin E acetic acid The ester selectivity is 98.3%. Stirring was stopped, and after standing for 3 minutes, the reaction solution was divided into two layers, the upper layer being the vitamin E acetate product phase, and the lower layer being the cocatalyst and solvent phase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com