Preparation and application of open-type hydrogen peroxide fluorescent probe compound
A technology of hydrogen peroxide and fluorescent probes, which is applied in the field of fluorescent probes, can solve the problems of few types of hydrogen peroxide probes, difficulty in detecting hydrogen peroxide, interference in hydrogen peroxide detection, etc., and achieve good sensitivity and selectivity, Good promotion prospect, good water solubility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
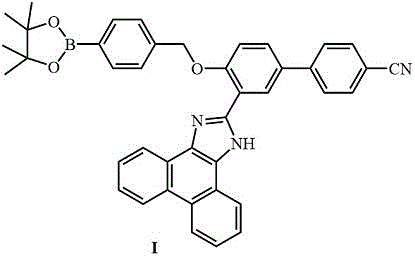
[0048] Embodiment 1, the synthesis of probe I
[0049] (a) In a nitrogen atmosphere, mix 6.24g (about 32mmol) of cyanobiphenol and 4.56g (about 48mmol) of anhydrous MgCl 2 In a three-neck round bottom flask, add 16.32mL (about 122mmol) of triethylamine, then add 80mL of anhydrous acetonitrile as a solvent, and finally add 12.3g (about 440mmol) of dry paraformaldehyde. The mixture was heated to 70 oC reflux for 8 hours. After the reaction was completed, it was cooled to room temperature, and then quenched with a small amount of water. Then add a large amount of 6M hydrochloric acid for acidification. The above crude product was treated with CH 2 Cl 2 For extraction (3*50 mL), the organic layer was washed with anhydrous MgSO 4 After drying, sample preparation and column chromatography purification, 3.5 g of white compound III was obtained with a yield of about 50%.
[0050] (b) Compound III (0.1g, 0.45mmol), bromobenzylboronate (0.133g, 0.45mmol), K 2 CO 3 (0.62g, 0.45m...
Embodiment 2
[0052] Embodiment 2, fluorescence experiment
[0053] Take the fluorescent probe compound prepared in Example 1, dissolve it in an aqueous solution containing 50% DMF, and adjust the pH to 7.4 with PBS buffer solution; obtain a fluorescent probe solution for future use.
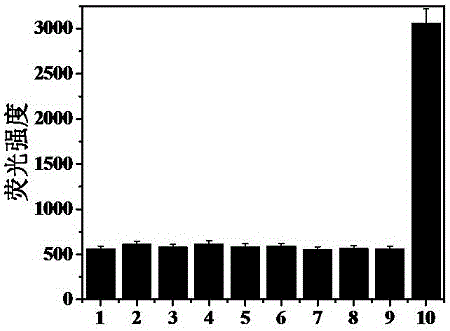
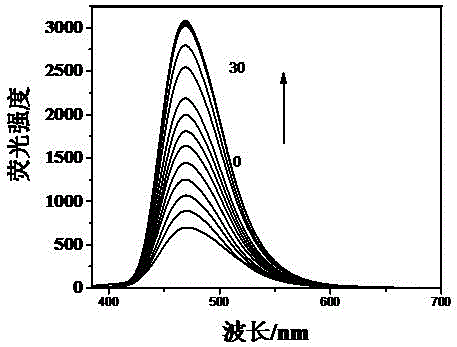
[0054] 1. Take the fluorescent probe solution and divide it into 12 groups, 10 ml in each group. Among them, 1 group does not add reactive oxygen species, and 11 groups add CH 3 COOOH, GSH, H 2 o 2 , HOCl, O 2 •- , •OH, otBU, TBHP, Vc solution, so that the concentration of the probe compound contained in each group of solutions is 10 μM, the concentration of the active oxygen species is 200 μM, so that the molar ratio of the active oxygen species to the probe compound is 30:1; The wavelength is 365nm, and the fluorescence intensity is tested by a fluorescence photometer, such as image 3 As shown, the results show that the probe solution of the present invention has no fluorescence itself. Once hydrogen ...
Embodiment 3
[0056] Embodiment 3, cell imaging experiment
[0057] MCF-7 cells were cultured in 1 milliliter of cell culture medium containing 10% bovine fetal serum for 12 hours, then treated with 100 micromoles per liter of fluoride ion for 10 minutes, and then treated with 10 micromoles per liter of the fluorescent probe of the present invention Process for 30 minutes. The cells were excited with a light source with an excitation wavelength of 365, and imaged under a confocal microscope, such as Figure 5 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com