Ofloxacin-hemocyanin coating antigen and preparation method thereof and detection test card
A technology of ofloxacin and hemocyanin, which is applied in the fields of biomedicine and medical testing, can solve the problems of inability to estimate the specificity of monoclonal antibodies, etc., and achieve the effects of easy large-scale promotion and application, quick results, and reduced error rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The invention provides a preparation method of ofloxacin-hemocyanin coating former, comprising the following steps:
[0045] 1) Carrying out dehydration condensation reaction of aminovaleric acid and ofloxacin in a buffer solution system, extracting and back-extracting the obtained reaction solution in sequence to obtain the ofloxacin hapten with carbon chain extension;
[0046]2) The carbon chain extended hapten ofloxacin hapten that described step 1) is obtained is dissolved in N, N-dimethylformamide, obtains the ofloxacin hapten solution that carbon chain extends; The ester is mixed with the carbon chain extended ofloxacin hapten solution, stirred and reacted to obtain activated ofloxacin hapten;
[0047] 3) mixing hemocyanin and phosphate buffer solution, adjusting the pH value of the mixture to 9.0-10, and reacting under shaking conditions to obtain hemocyanin;
[0048] 4) dissolving the activated hemocyanin obtained in step 3) in a phosphate buffer solution conta...
Embodiment 1
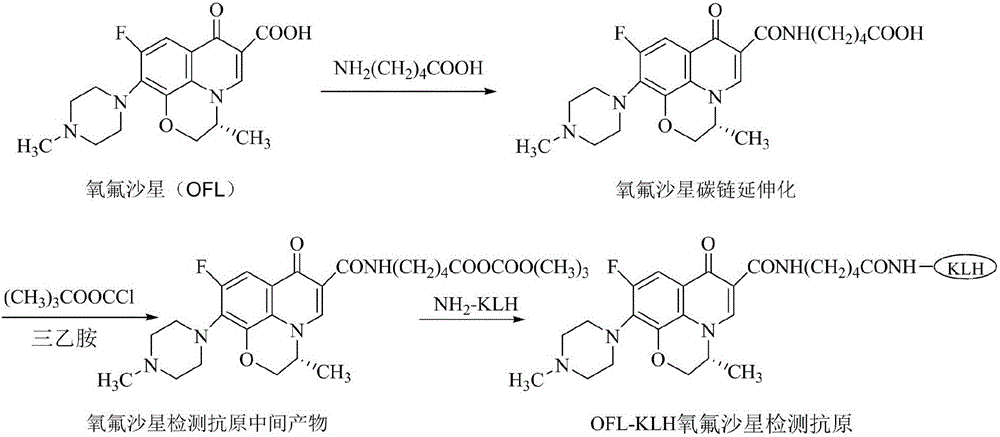
[0108] Ofloxacin detects the synthesis of antigen, and the synthesis reaction is as attached figure 1 shown.
[0109] Ofloxacin carboxyl C chain extension: Weigh 2mmol of aminovaleric acid and add it to the Erlenmeyer flask, then adjust the pH value with 2ml of sodium hydroxide solution, stir in an ice bath; dissolve ofloxacin in methanol containing 50% DMF, stir In the state, add aminovaleric acid-sodium hydroxide solution drop by drop, and react with magnetic stirring for 2 hours; extract with ethyl acetate, wash with dilute hydrochloric acid, and back-extract with sodium bicarbonate solution to obtain the OFL hapten.
[0110] The detection antigen of ofloxacin-hemocyanin (OFL-KLH) is realized by the mixed acid anhydride coupling method: Weigh 20 mg OFL hapten and dissolve it in 2 ml DMF, add 10 μL triethylamine, and put it in an ice bath at 4°C Reaction 1h. Then 30 μL of isobutyl chloroformate was added, and the stirring reaction was continued for 1 h. 30mg of KLH was di...
Embodiment 2
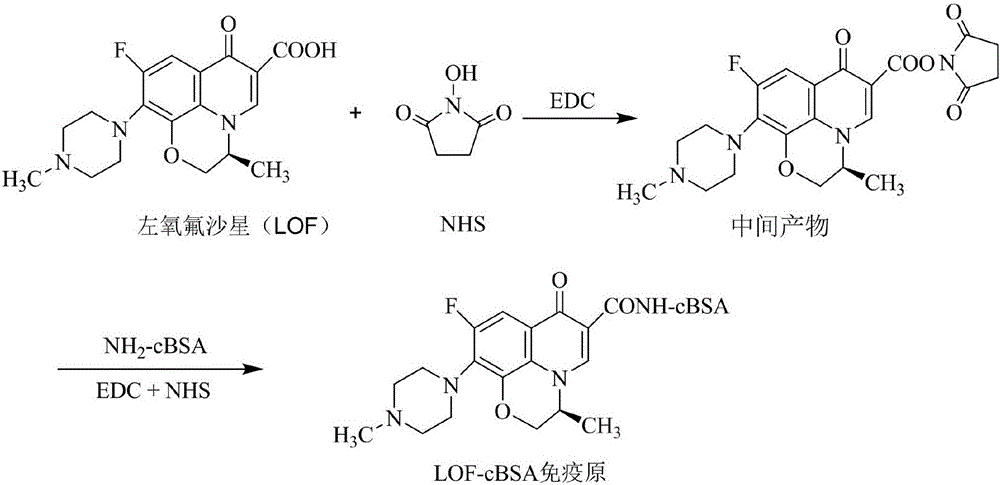
[0112] Synthesis of levofloxacin (LOF) immunogen; Synthetic reaction as attached figure 2 shown.
[0113] (1) BSA activation: Glycosylation modification method, in which the aldehyde group on the glucose is coupled to the carboxyl group on the protein in an alkaline environment, the carboxyl group is blocked, and the number of coupled amino groups on the BSA is increased. The specific process is as follows: Weigh 66 mg of bovine serum albumin (BSA) and 1 mg of glucose and dissolve them in PBS buffer, adjust the pH value to 9.5 with NaHCO3, and react on a shaker at 40° C. for 2 hours to obtain activated cBSA.
[0114] (3) Synthesis of immunogen: LOF immunogen was synthesized by a two-step carbodiimide method. Take 19.3mg of LOF hapten, stir and dissolve with 2.3ml N,N-dimethylformamide (DMF), then add 14.4mg of N-hydroxysuccinimide (NHS) and 1-ethyl-(3-dimethyl Aminopropyl) carbodiimide hydrochloride (EDC.HCl) 51.2 mg, dissolved at room temperature, protected from light, sha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com