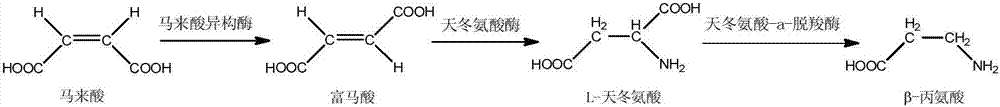
Method for preparing beta-alanine from maleic acid through multi-enzyme coupling
A technology of maleic acid isomerase and maleic acid, which is applied in the biological field, can solve the problems of low yield of repeated purification, high safety requirements of the production site, and high production cost, and achieve reduced production costs, low cost, and low substrate fully transformed effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Get Alcaligenes faecalis wet thallus 5g with maleic acid isomerase activity, Escherichia coli wet thallus 0.5g with aspartic acid-alpha-decarboxylation Add 10 g of enzyme-active Corynebacterium glutamicum wet thallus into 1000 ml of transformation solution containing 50 g / L maleic acid, 0.01 g / L pyridoxal phosphate and 0.01 g / L Tween 80, pH 6.0, 30 ℃ enzymatic reaction 15h, the reaction is terminated. After the reaction, the concentration of β-alanine in the conversion liquid was 36.1 g / L, and the molar conversion rate to maleic acid was 94%.
[0041] 2. Centrifuge the transformation solution at 4000r / min for 15min to remove the bacteria, heat the supernatant to 70-80°C, add activated carbon for decolorization, the decolorization solution is purified by ultrafiltration and nanofiltration, the purified solution is vacuum concentrated to 40ml, cooled and crystallized to room temperature , suction filtration, and drying to obtain 14 g of solid β-alanine.
[0042]3. Af...
Embodiment 2
[0044] 1. Get Alcaligenes faecalis wet thallus 5g with maleic acid isomerase activity, Escherichia coli wet thallus 0.5g with aspartic acid-alpha-decarboxylation Add 15g of enzyme-active Corynebacterium glutamicum wet cells into 1000ml of transformation solution containing 100g / L maleic acid, 0.1g / L pyridoxal phosphate and 0.1g / L TritonX 100, pH 7.0 , 37 ℃ enzymatic reaction 20h, the reaction is terminated. After the reaction, the concentration of β-alanine in the conversion solution was 72.9 g / L, and the molar conversion rate to maleic acid was 95%.
[0045] 2. Centrifuge the transformation solution at 4000r / min for 15 minutes to remove the bacteria, heat the supernatant to 70-80°C, add activated carbon for decolorization, the decolorization solution is purified by ultrafiltration and nanofiltration, the purified solution is vacuum concentrated to 80ml, cooled and crystallized to room temperature , suction filtration, and drying to obtain 29 g of solid β-alanine.
[0046] 3...
Embodiment 3
[0048] 1. Get Alcaligenes faecalis wet thallus 10g with maleic acid isomerase activity, Escherichia coli wet thallus 1.0g with aspartic acid-alpha-decarboxylation Add 20g of wet cells of Corynebacterium glutamicum with enzymatic activity to 1000ml transformation solution containing 100g / L maleic acid, 0.1g / L pyridoxal phosphate and 0.1g / L CTAB, pH 8.0 , 42 ℃ enzymatic reaction 18h, the reaction is terminated. After the reaction, the concentration of β-alanine in the conversion liquid was 73.6 g / L, and the molar conversion rate to maleic acid was 96%.
[0049] 2. Centrifuge the transformation solution at 4000r / min for 15 minutes to remove the bacteria, heat the supernatant to 70-80°C, add activated carbon for decolorization, the decolorization solution is purified by ultrafiltration and nanofiltration, the purified solution is vacuum concentrated to 80ml, cooled and crystallized to room temperature , suction filtration, and drying to obtain 29.5 g of solid β-alanine.
[0050]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com