Alginate medical dressing and preparation method thereof
A technology of alginate and alginate fiber, applied in the field of medical supplies, can solve the problems of single function, difficult to replace, and no anti-sticking ingredients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
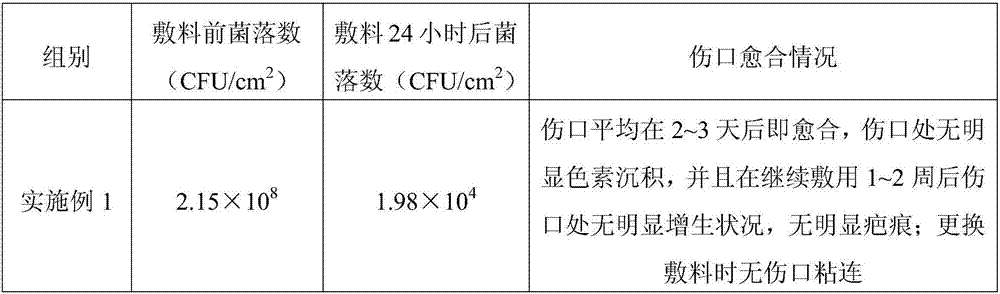
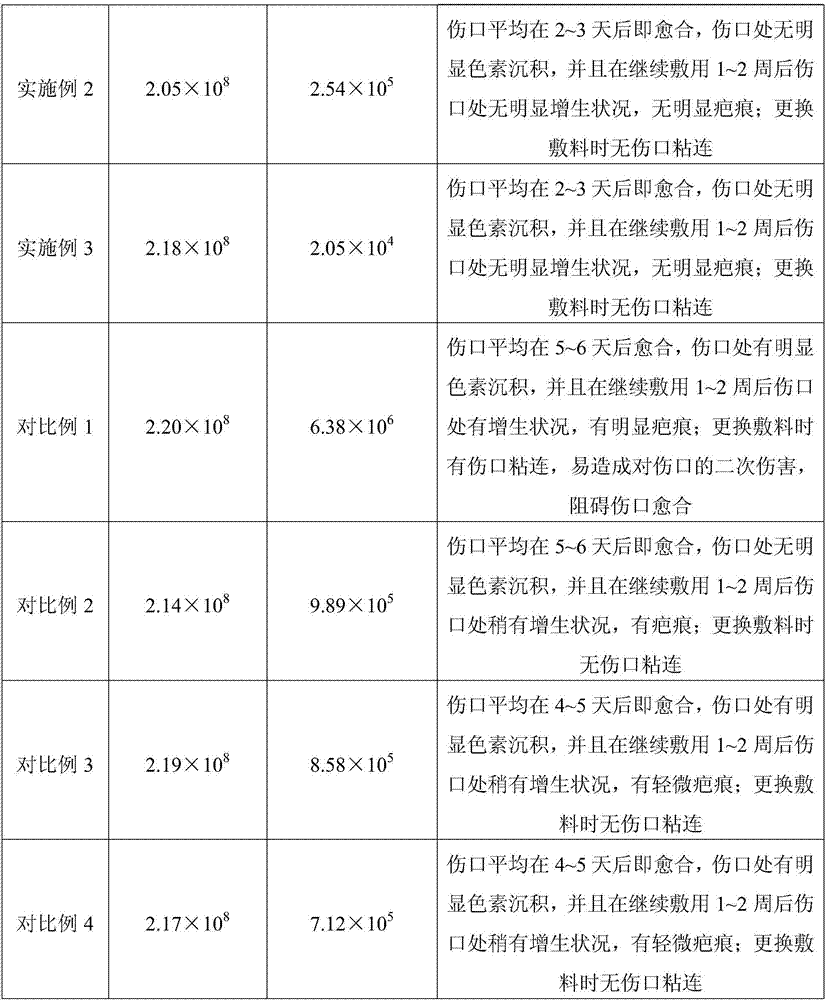
Embodiment 1
[0022] An alginate medical dressing prepared by soaking alginate fibers in a functional soaking liquid; wherein the functional soaking liquid is prepared from the following raw materials in percentage by weight: carboxymethyl chitosan 3% , chitosan oligosaccharide 1.5%, chitosan hydrochloride 5%, hyaluronic acid chitosan 1.5%, antibacterial agent 0.04%, sodium carboxymethyl cellulose 3%, calcium chloride 3%, epidermal growth factor 0.03%, aloe vera extract 0.04%, and water as the balance; the mass dosage ratio of alginate fiber and functional soaking liquid is 1:30.
[0023] The preparation method of above-mentioned alginate medical dressing comprises the following steps:
[0024] 1) Take carboxymethyl chitosan, chitosan oligosaccharide, chitosan hydrochloride, hyaluronic acid chitosan, antibacterial agent, sodium carboxymethyl cellulose, calcium chloride, epidermal growth factor, aloe extract The substance is added into water, mixed evenly, and a functional soaking liquid is...
Embodiment 2
[0027] An alginate medical dressing prepared by soaking alginate fibers in a functional soaking solution; wherein the functional soaking solution is prepared from the following raw materials in percentage by weight: wherein the functional soaking solution consists of the following weight Prepared from the raw materials used in percent: carboxymethyl chitosan 2%, chitosan oligosaccharide 1%, chitosan hydrochloride 3%, hyaluronic acid chitosan 1%, antibacterial agent 0.03, carboxymethyl Sodium cellulose 0.5%, calcium chloride 1%, epidermal growth factor 0.005%, aloe extract 0.02%, and water as the rest; the mass ratio of alginate fiber to functional soaking solution is 1:50.
[0028] The preparation method of above-mentioned alginate medical dressing comprises the following steps:
[0029] 1) Take carboxymethyl chitosan, chitosan oligosaccharide, chitosan hydrochloride, hyaluronic acid chitosan, antibacterial agent, sodium carboxymethyl cellulose, calcium chloride, epidermal gro...
Embodiment 3
[0032] An alginate medical dressing prepared by soaking alginate fibers in a functional soaking solution; wherein the functional soaking solution is prepared from the following raw materials in percentage by weight: wherein the functional soaking solution consists of the following weight It is prepared from the raw materials in percent dosage: 4% carboxymethyl chitosan, 2% chitosan oligosaccharide, 6% chitosan hydrochloride, 2% hyaluronic acid chitosan, 0.05% antibacterial agent, carboxymethyl chitosan Sodium cellulose 5%, calcium chloride 6%, epidermal growth factor 0.05%, aloe extract 0.05%, the rest is water; the mass ratio of alginate fiber to functional soaking solution is 1:10.
[0033] The preparation method of above-mentioned alginate medical dressing comprises the following steps:
[0034] 1) Take carboxymethyl chitosan, chitosan oligosaccharide, chitosan hydrochloride, hyaluronic acid chitosan, antibacterial agent, sodium carboxymethyl cellulose, calcium chloride, ep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com