Thiophene oxide derivative and organic light-emitting device thereof
A technology of oxides and derivatives, applied in the field of thiophene oxide derivatives and their organic light-emitting devices, can solve the problems of electron mobility slower than hole mobility, triplet energy quenching, efficiency roll-off, etc., to achieve luminescence Efficiency enhancement, solution to carrier imbalance, effect of high triplet energy level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
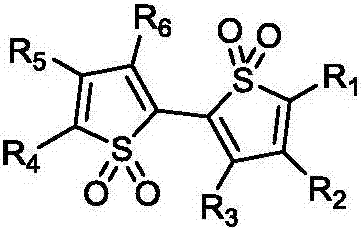
[0033] The preparation method of the thiophene oxide derivatives described in the present invention can obtain compound A by bromination of thiophene, and then obtain compound B through coupling reaction, compound B obtains compound C through a series of bromination and coupling reactions, compound C is oxidized to obtain the target compound.
[0034]
[0035] The present invention also provides an organic light-emitting device, comprising a first electrode, a second electrode, and one or more organic compound layers placed between the two electrodes, at least one of the organic compound layers containing the present Invention of the thiophene oxide derivatives. The organic compound layer includes at least one of a hole injection layer, a hole transport layer, an electron blocking layer, a light emitting layer, a hole blocking layer, an electron transport layer, and an electron injection layer.
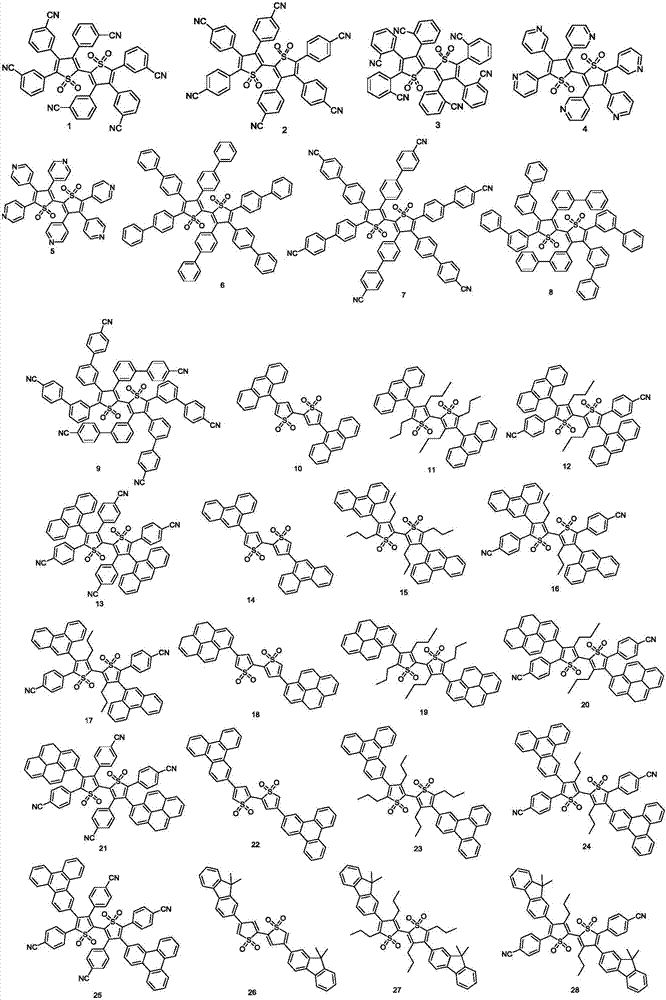
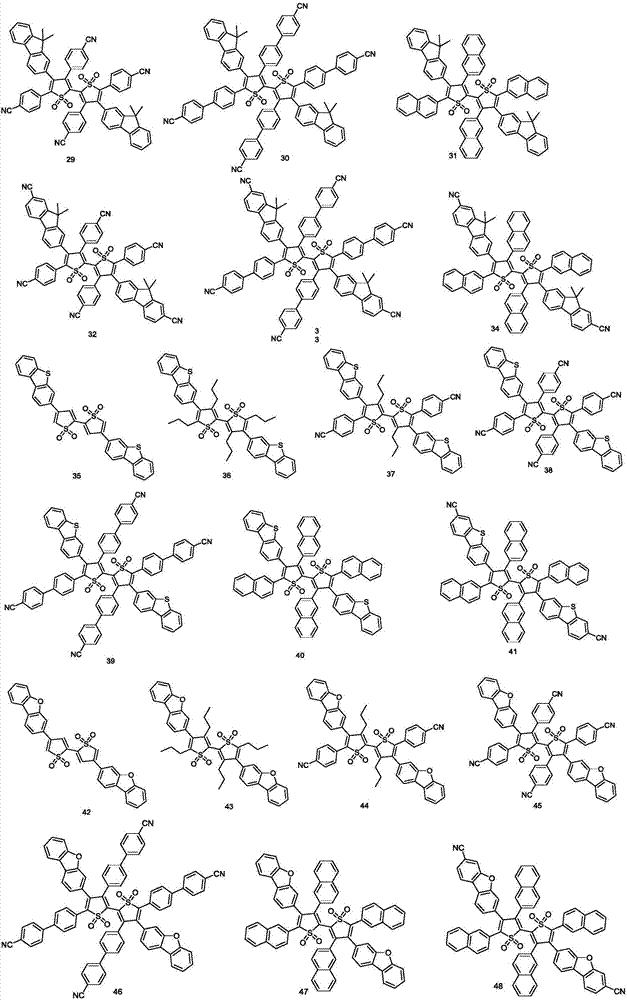
[0036] The thiophene oxide derivative provided by the present invention can e...
Embodiment 1
[0040] [Example 1] Synthesis of compound 2
[0041]
[0042] *Synthesis of intermediate 2-1
[0043] The three-necked flask of the reactor was placed in an ice bath, and thiophene (8.4g, 0.1mol), 20mL of CCl 4 , control the temperature at 0-5°C, stir and add dropwise 4.8mL of bromine (15g, 0.115mol) and 19mL of hydrobromic acid (23.5g, 0.116mol), the dropwise addition is completed within 80min, and then stirred for 60min, then washed with water and dried 1. The solvent was recovered by atmospheric distillation, the product was transferred into a distillation flask, and the fraction at 58-62° C. / 7.33 KPa was collected to obtain a colorless liquid 2-1 (14.3 g, 88%).
[0044] *Synthesis of intermediate 2-2
[0045] The reactor was put into an ice bath, and 20 mL of THF, compound 2-1 (3.26 g, 20 mmol), NiCl 2 (dppp) (0.54g, 1mmol), the temperature was controlled at 0-5°C, and then a solution of 2-thiophene magnesium bromide (4.5g, 24mmol) dissolved in 20mLTHF was added dropw...
Embodiment 2
[0050] Under the protection of argon, 20ml of H 2 o 2 Slowly added dropwise to 2-4 (6.73, 8.4mmol) in a mixed solvent of acetic acid (60ml) and DCM (25ml). After the reaction system was reacted at room temperature for 1 h, it was heated to 90°C and reacted for 36 h. After the initial product of the reactant was extracted with DCM, it was separated by column to obtain compound 2 (3.71 g, 53%). [Example 2] Synthesis of compound 4
[0051] According to the synthetic method of compound 2, compound 4 (2.91 g, 50%) was obtained.
[0052]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com