Application of 5-pentyl-3-methoxy-phenol in the preparation of oxidative stress or inflammatory response-induced disease prevention products
A technology of oxidative stress and inflammatory response, applied in the field of medicine, can solve the problems of less research on the chemical constituents of P. japonicus and the evaluation of the biological activity of chemical constituents.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0033] A typical embodiment of the present application provides the use of 5-pentyl-3-methoxy-phenol in the preparation of oxidative stress or inflammatory response-induced disease prevention products, the 5-pentyl-3-methoxy-phenol The chemical formula of the base-phenol is:
[0034]
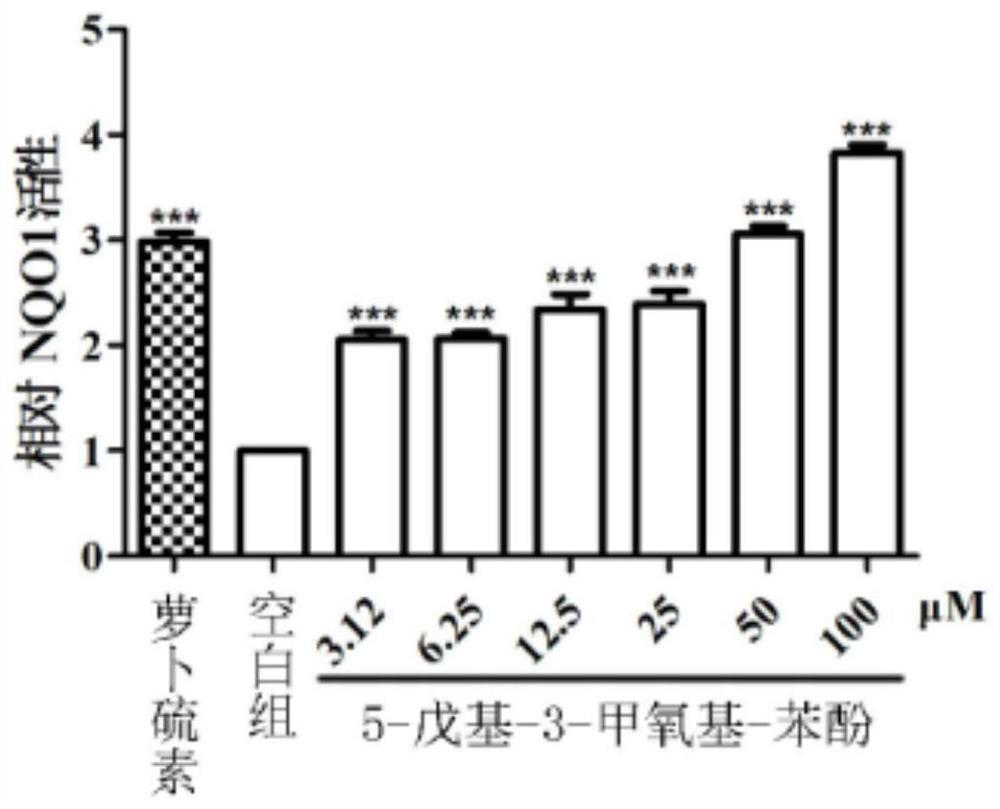
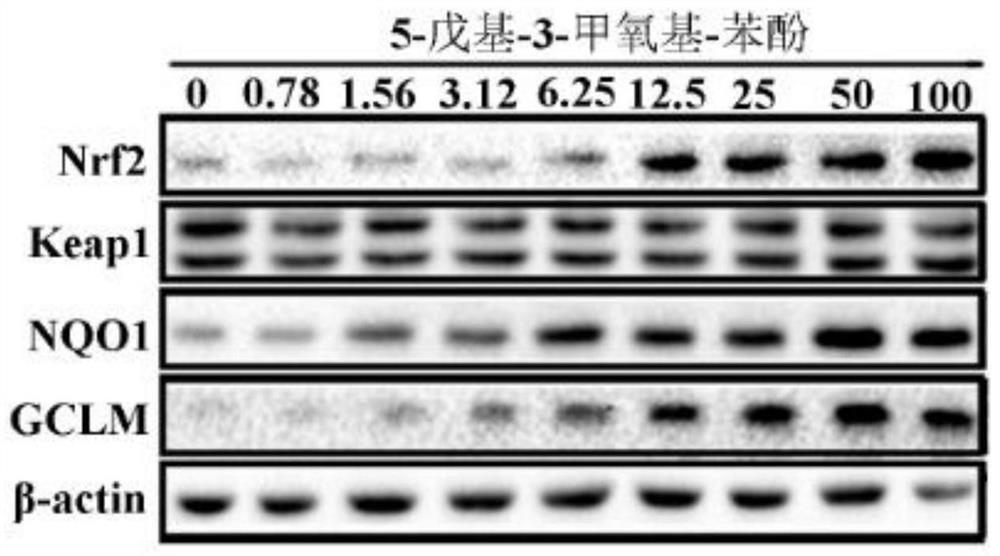
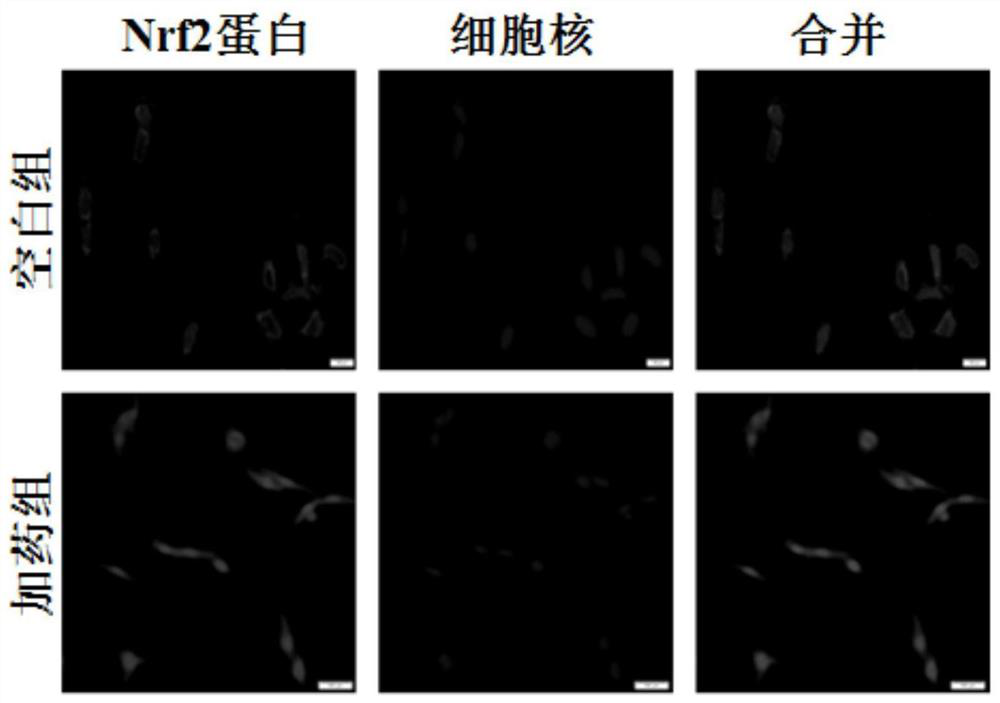
[0035] The present application found that 5-pentyl-3-methoxy-phenol can upregulate the protein levels of Nrf2 and its phase II detoxification enzyme NQO1 and antioxidant enzyme GCLM, and inhibit the production of excessive endogenous reactive oxygen species induced by arsenic. , its activation mechanism is achieved by increasing the stability of Nrf2 protein and inhibiting the degradation of Nrf2 protein; at the same time, down-regulating the excess endogenous reactive oxygen species induced by arsenic can also weaken the activation of NF-κB signaling pathway, down-regulate the expression of inflammatory genes, and protect the body from the environment hazards of contaminants. In order to co...
Embodiment 1
[0055] Example 1: Structural confirmation of 5-pentyl-3-methoxy-phenol
[0056] The aerial part of Ginger japonica in southern Yunnan was extracted with 95% ethanol to obtain an ethanol extract, which was then extracted with petroleum ether, ethyl acetate and n-butanol in sequence. The petroleum ether part adopts petroleum ether-ethyl acetate system gradient elution, and the concentrations are respectively pure petroleum ether, petroleum ether-ethyl acetate=95:5 (volume ratio), petroleum ether-ethyl acetate=90:10 (volume ratio) ), petroleum ether-ethyl acetate=85:15 (volume ratio), petroleum ether-ethyl acetate=80:20 (volume ratio), petroleum ether-ethyl acetate=75:25 (volume ratio), petroleum ether- Ethyl acetate=70:30 (volume ratio), petroleum ether-ethyl acetate=60:40 (volume ratio), petroleum ether-ethyl acetate=50:50 (volume ratio), petroleum ether-ethyl acetate=40 : 60 (volume ratio), 100% ethyl acetate to obtain 14 fractions (Frs. 1-14). Part Fr.8 (petroleum ether-ethyl...
Embodiment 2
[0059] Example 2: Evaluation of NQO1-inducing activity of 5-pentyl-3-methoxy-phenol
[0060] (1) Culture of mouse hepatoma cell hepa 1c1c cell line
[0061] The mouse hepatoma cell hepa 1c1c cell line was purchased from the American Type Culture Collection (ATCC) in MEM medium containing 10% fetal bovine serum (FBS), placed at 37°C, 5% CO 2 Cultivated in an incubator.
[0062] (2) NQO1 induction activity test
[0063] Hepa 1c1c cells were seeded on 96-well plates, and after the cells adhered, different concentrations of 5-pentyl-3-methoxy-phenol (confirmed in Example 1) were added, treated for 24 hours, and lysed with 0.8% digitonin solution. Cells, add detection solution (1.0mL 0.5M Tris-HCl, 15mg BSA, 6mg MTT, 150μL Tween-20, 150μL 150mM D-glucose-6-phosphate, 15μL 7.5 mM flavin adenine dinucleotide, 27 μL of 50 mM nicotinamide adenine dinucleotide phosphate, 20 μL of 50 mM menadione), left for 3 minutes, and the luminescence intensity was measured at 630 nm.
[0064] Re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com