Rhizomucor miehei-derived beta-1,3-glucanase and application thereof
A technology of glucanase and seeds, applied in the field of β-1,3-glucanase, to achieve good enzymatic properties and good application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1. Cloning of β-1,3-glucanase gene and expression in Pichia pastoris
[0043] Design upstream primer RmLam81AEcoRI (5'-attCCG GAATTC CAGAGTACAAGTGATGGAGACGAT-3', the underline shows the EcoRI restriction site) and the downstream primer RmLam81ANotI (5'-ATAAGAAT GCGGCCGC TCAGCGACGGAAGAAATGAGGAC-3', the underline shows the NotI restriction site), and the amino acid sequence of the encoded mature protein was amplified by PCR using the genomic DNA of Rhizomucor miehei CAU432 as a template. The amplification conditions were: pre-denaturation at 94°C for 5 min; denaturation at 94°C for 30 s, annealing at 54°C for 30 s, extension at 72°C for 150 s, and 35 cycles; finally, extension at 72°C for 5 min. The product was recovered by 1% agarose gel electrophoresis and digested with EcoRI and NotI. Ligate the digested product with the same digested expression vector pPIC9K with T4 DNA ligase, transform E.coli DH5α competent cells by heat shock, spread on LB plates contai...
Embodiment 2
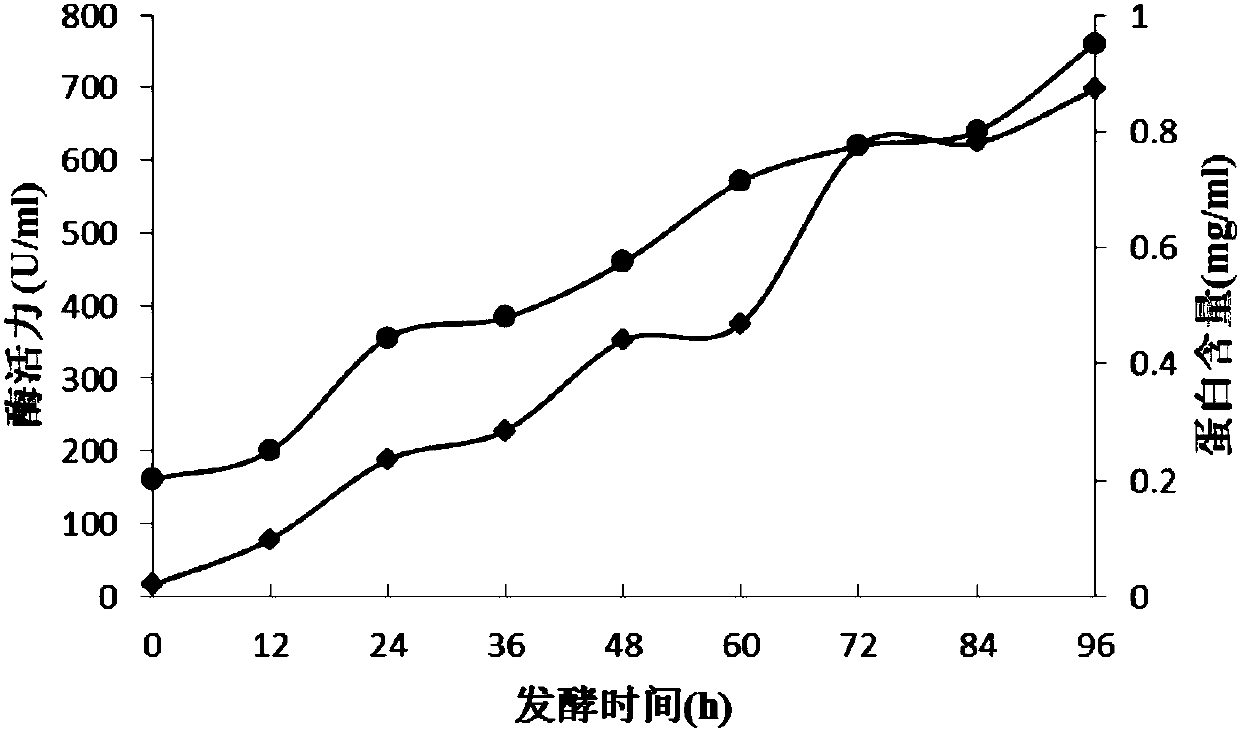
[0046] Example 2. High-density fermentation and purification of recombinant β-1,3-glucanase
[0047] 1. High-density fermentation of recombinant β-1,3-glucanase
[0048] Seed cultivation: Inoculate the recombinant Pichia strain A obtained in Experimental Example 1 into a 500mL Erlenmeyer flask filled with 100mL of BMGY medium, and vibrate at 30°C and 200rpm for 24-30h to obtain a seed solution with an OD600nm of about 3.0.
[0049] Glycerol fermentation stage: inoculate in 5L fermenter (2L BMGY culture medium is housed, solute is the material of following final concentration: the yeast extract that mass percent concentration is 1%, the peptone that mass percent concentration is 2%, 100mmol / L pH 6.0 phosphate buffer solution, the mass percent concentration is 1.34% YNB, and the mass percent concentration is 4×10 -5 % biotin, 1% glycerol by volume). Temperature in the process is 30 DEG C, adjust pH to 5.0 with ammoniacal liquor and phosphoric acid, control dissolved oxygen to...
Embodiment 3
[0055] Example 3. Properties of recombinant β-1,3-glucanase (RmLam81A)
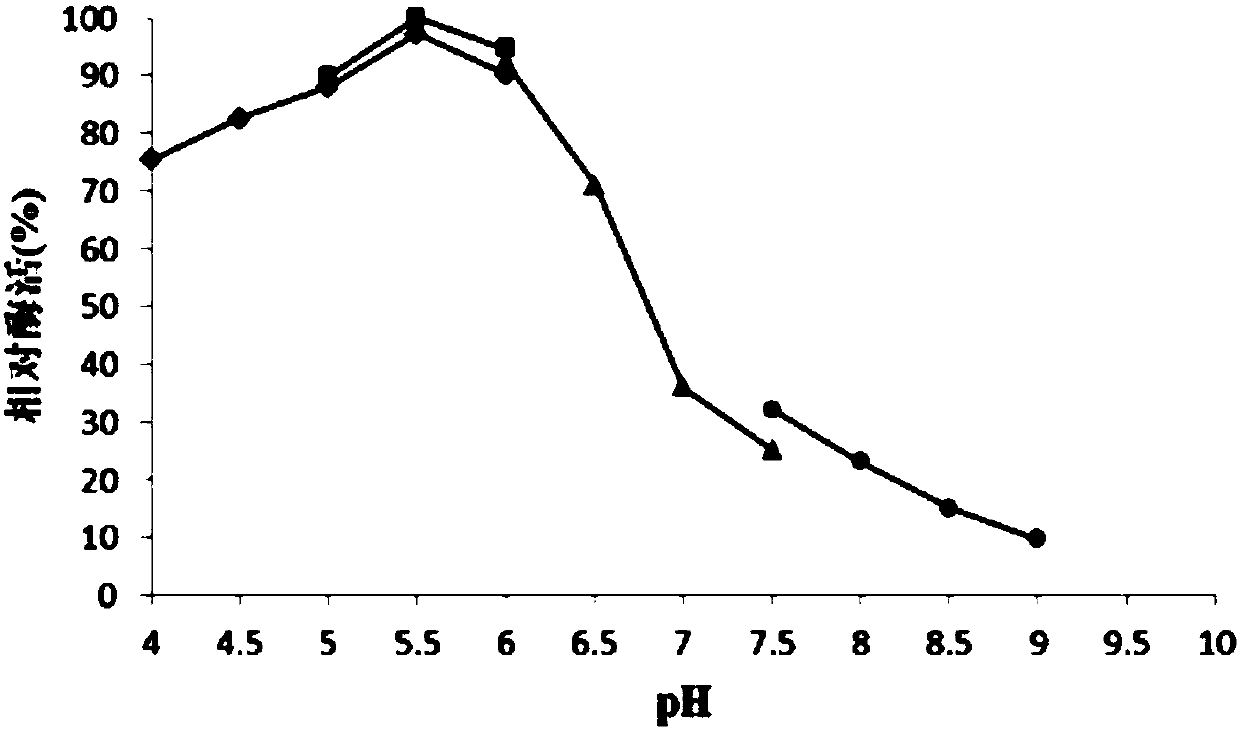
[0056] 1. Optimum pH determination of RmLam81A
[0057] The optimal pH value of the enzyme was determined with the following four buffer systems with different pH values: acetic acid-sodium acetate buffer (pH4.0-6.0); citric acid-sodium citrate buffer (pH5.0-6.0); Phosphate buffer (pH6.0-7.5); Tris-HCl buffer (pH7.5-9.0). Then adopt the standard method to measure the enzyme activity of β-1,3-glucanase at 40 ℃, take the highest value of the enzyme activity as 100%, and calculate the relative enzyme activities measured at each pH respectively, the results are as follows image 3 shown. image 3 It was shown that the optimum pH value of recombinant β-1,3-glucanase (RmLam81A) was 5.5 (citric acid-sodium citrate buffer).
[0058] 2. Determination of pH stability of RmLam81A
[0059] Dilute the RmLam81A enzyme solution with the above four buffer solutions of different pH, keep the diluted enzyme solution in a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com