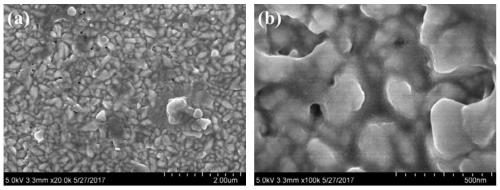
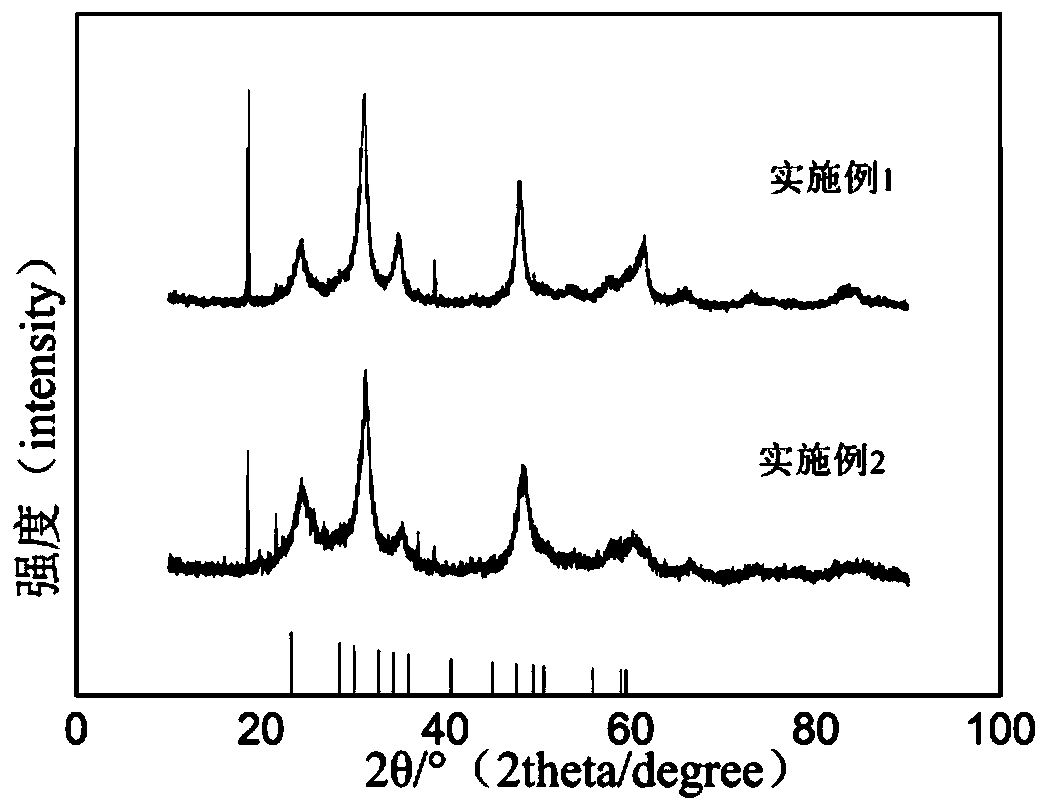
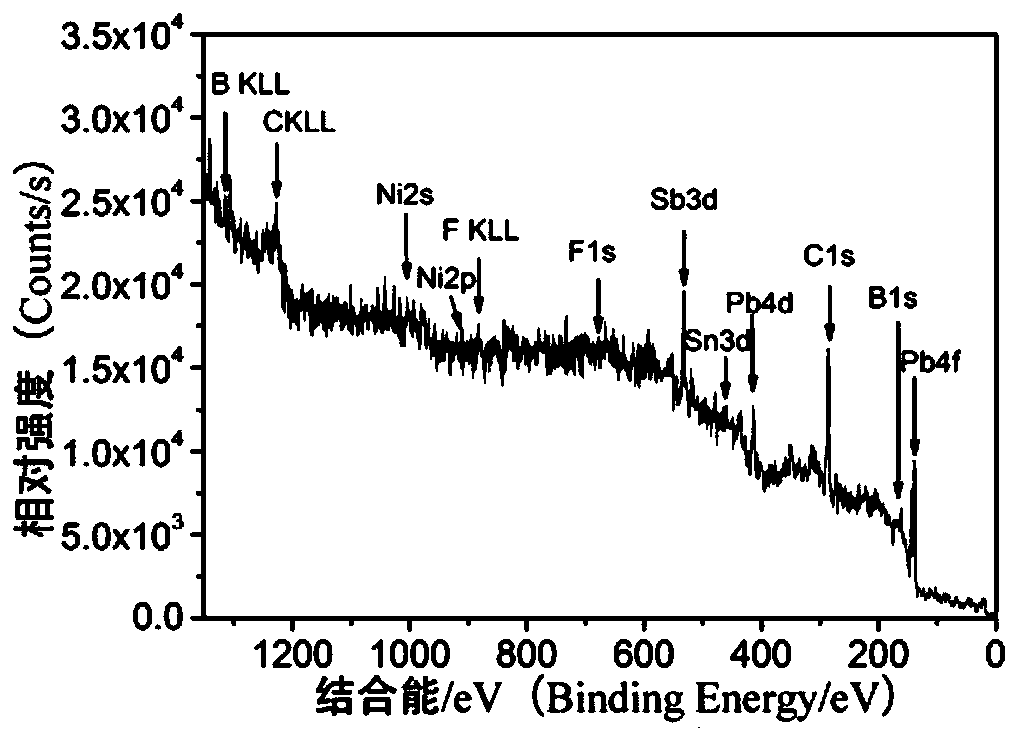
A nickel-boron-fluorine co-doped lead dioxide anode and its preparation method and application
A technology of lead dioxide and co-doping, which is applied in chemical instruments and methods, oxidized water/sewage treatment, water/sludge/sewage treatment, etc., can solve the problem of unsatisfactory electrode current efficiency and electrode service life, and chemical stability It needs to be further improved, the preparation process conditions are harsh, etc., to achieve the effect of improving electrode stability, stable mechanical properties, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] A method for preparing a nickel-boron-fluorine co-doped lead dioxide anode, comprising the following steps:
[0054] (1) The porous titanium plate is placed in a hydrochloric acid solution (the volume ratio of concentrated hydrochloric acid and water is 1:2) and boiled for 15min, then, ultrasonically (ultrasonic power 35KHz) in distilled water is cleaned for 8min to obtain a pretreated porous titanium plate; Citric acid, ethylene glycol, tin tetrachloride and antimony trichloride are mixed, heated and stirred to obtain a molten sol; wherein, the molar ratio of citric acid, ethylene glycol, tin tetrachloride and antimony trichloride is 650 :200:9:1;
[0055] (2) Coating the molten sol obtained in step (1) on the pretreated porous titanium plate, drying at 140°C for 10 minutes, then calcining at 550°C for 10 minutes, and cooling to 30°C; repeat coating-drying-calcination- Cooled for 5 times, calcined again at 550 °C for 1 h to obtain a tin-antimony bottom layer;
[0056...
Embodiment 2
[0061] A method for preparing a nickel-boron-fluorine co-doped lead dioxide anode, comprising the following steps:
[0062] (1) The porous titanium plate is placed in a hydrochloric acid solution (the volume ratio of concentrated hydrochloric acid and water is 1:2), boiled for 20 minutes, and ultrasonically cleaned with distilled water (ultrasonic power 40KHz) for 10 minutes to obtain a pretreated porous titanium plate; , ethylene glycol, tin tetrachloride and antimony trichloride are mixed, heated and stirred to obtain a molten sol; wherein, the molar ratio of citric acid, ethylene glycol, tin tetrachloride and antimony trichloride is 700:100 : 10:2;
[0063] (2) Coating the molten sol obtained in step (1) on the pretreated porous titanium plate, drying at 130°C for 20min, calcining at 600°C for 20min, cooling to 20°C, repeating coating-drying-calcination -Cool 6 times, calcined again at 600°C for 1.5h to obtain the tin-antimony bottom layer;
[0064] (3) the tin-antimony b...
Embodiment 3
[0068] A method for preparing a nickel-boron-fluorine co-doped lead dioxide anode, comprising the following steps:
[0069] (1) The porous titanium plate is placed in a hydrochloric acid solution (the volume ratio of concentrated hydrochloric acid and water is 1:2) and boiled for 18min, then, ultrasonically (ultrasonic power 30KHz) in distilled water is cleaned for 5min to obtain a pretreated porous titanium plate; Citric acid, ethylene glycol, tin tetrachloride and antimony trichloride are mixed, heated and stirred to obtain a molten sol; wherein, the molar ratio of citric acid, ethylene glycol, tin tetrachloride and antimony trichloride is 600 :150:8:1.5;
[0070] (2) Coating the molten sol obtained in step (1) on the pretreated porous titanium plate, drying at 135°C for 15min, then calcining at 500°C for 15min, and cooling to 40°C; repeat coating-drying-calcination- Cooling for 7 times, calcining at 500 °C for 2 h, to obtain the tin-antimony bottom layer;
[0071] (3) the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com