Alanine dehydrogenase mutant and application thereof
A technology of alanine dehydrogenase and mutants, which is applied in the field of alanine dehydrogenase mutants and its application, and can solve the problems of reduced enzyme activity of alanine dehydrogenase and unfavorable L-alanine production, etc. , to achieve the effect of efficient production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Acquisition of Thermophilic Archaeal Alanine Dehydrogenase Gene
[0028] (1) Inoculate the purchased thermophilic archaea in nutrient broth medium, collect the bacteria after culturing at 80°C for 10 hours, and extract genomic DNA using a bacterial genome extraction kit;
[0029] (2) using primers alaD1 (5'ATGGAGACTCTTATTTTGACTCAGG 3', SEQ ID NO.5) and alaD2 (5'TCATATCCTGAAAAACTTTATTTTA3', SEQ ID NO.6) to clone the AFalaD gene encoding alanine dehydrogenase from genomic DNA;
[0030] (3) Connect the gene to the PMD19 simple cloning vector for sequencing to obtain a gene sequence such as SEQ ID NO.1;
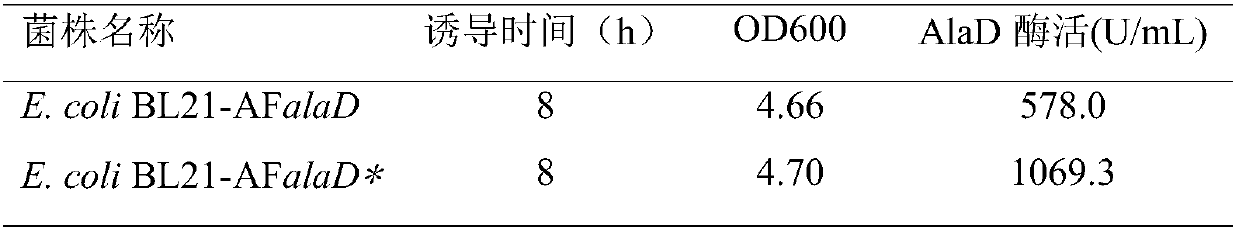
[0031] (4) The AFalaD* gene was obtained after codon optimization of SEQ ID NO.1 according to the codon expression preference of the E.coli gene. The gene sequence is as shown in SEQ ID NO.2. After optimization, the GC content of the gene was reduced from 50.5% to 48.9 %, the codon adaptation index (CAI) increased from 0.222 to 0.974;
[0032] (5) The genes AF...
Embodiment 2
[0037] Example 2: Protein engineering of thermophilic archaeal alanine dehydrogenase
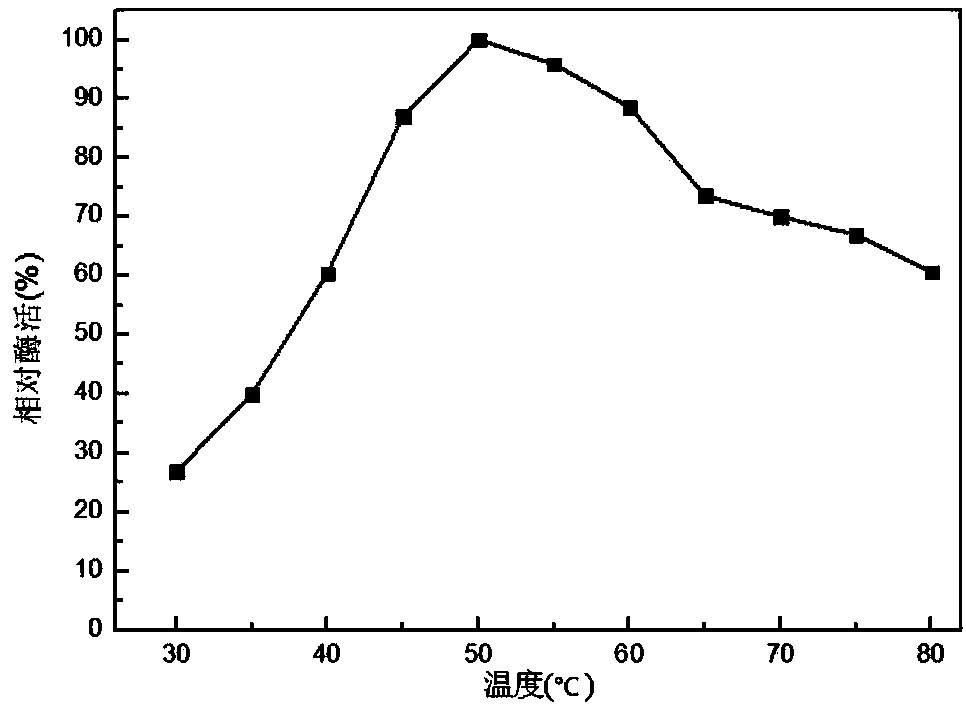
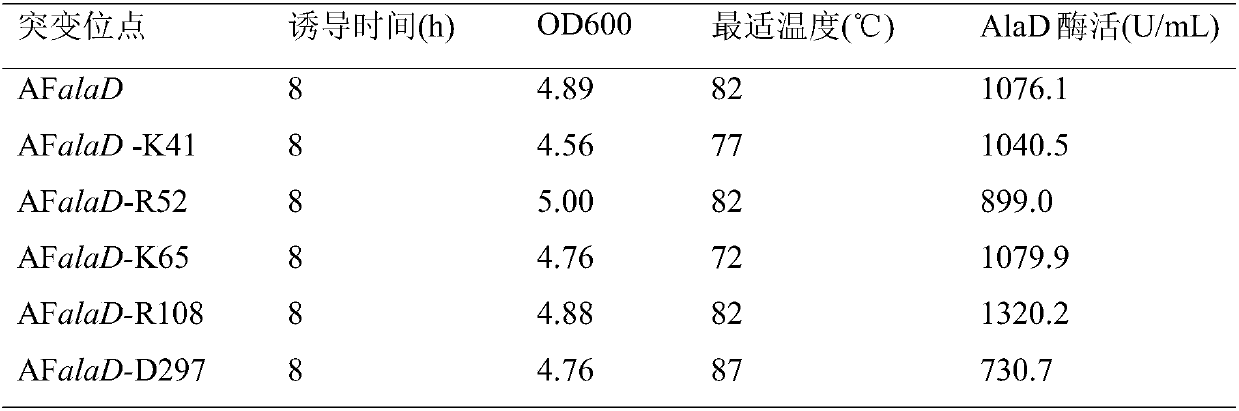
[0038] (1) Using Modeller software to model the homology of AFAlaD protein (JMB 342, 119-130 (2004)), the key amino acids K41, R52, K65, R108, and D297 of the loop structure of the catalytic active center of AFalaD* gene were mutated into Alanine; After the mutation, construct the recombinant strain according to the method in Example 1, and carry out enzyme activity detection, the results are shown in Table 2, the 41st lysine K and the 65th lysine K are mutated into alanine A, AFAlaD The optimum temperature for enzyme activity decreased to 77°C and 72°C respectively, so two sites were selected for combined mutation.
[0039] Table 2 Determination of enzyme activity of different mutants
[0040]
[0041] (2) Combining mutations of K41 and K65, it is found that when the 41st lysine K is mutated to histidine H, and the 65th lysine K is mutated to tryptophan W, that is, the 122nd base in SEQ...
Embodiment 3
[0042] Embodiment 3: the construction of high-yield L-alanine Escherichia coli
[0043] Using E.coli K12 as the starting strain, the key genes in the synthesis pathway of acetic acid, formic acid, ethanol, succinic acid, and lactic acid metabolites were knocked out by the Red homologous recombination method: acetate kinase gene ack-pta, pyruvate formate lyase gene pflB , alcohol dehydrogenase gene adhE, fumarate reductase gene frdA, fermentative D-lactate dehydrogenase gene ldhA, to obtain E.coliΔ5 strain, and integrate and express the AFalaD** gene derived from thermophilic archaea after protein engineering In E.coliΔ5, the genes encoding L-alanine dehydrogenase alaD and alanine racemase dadX were replaced to obtain engineering strain E.coliΔ5D2. The recombinant strain culture does not require the addition of antibiotics or inducers.
[0044] The above method adopts the Red homologous recombination system method:
[0045] 1) Introduce DNA fragment 1 with homology arm and ka...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com