Preparation method of potassium ion battery positive electrode material potassium vanadium fluorophosphates/carbon
A technology of potassium vanadium fluorophosphate and battery positive electrode, which is applied in battery electrodes, positive electrodes, secondary batteries, etc., can solve the problems of low diffusion kinetic performance, and achieve the effects of high conductivity, avoidance of coexistence and uniform particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
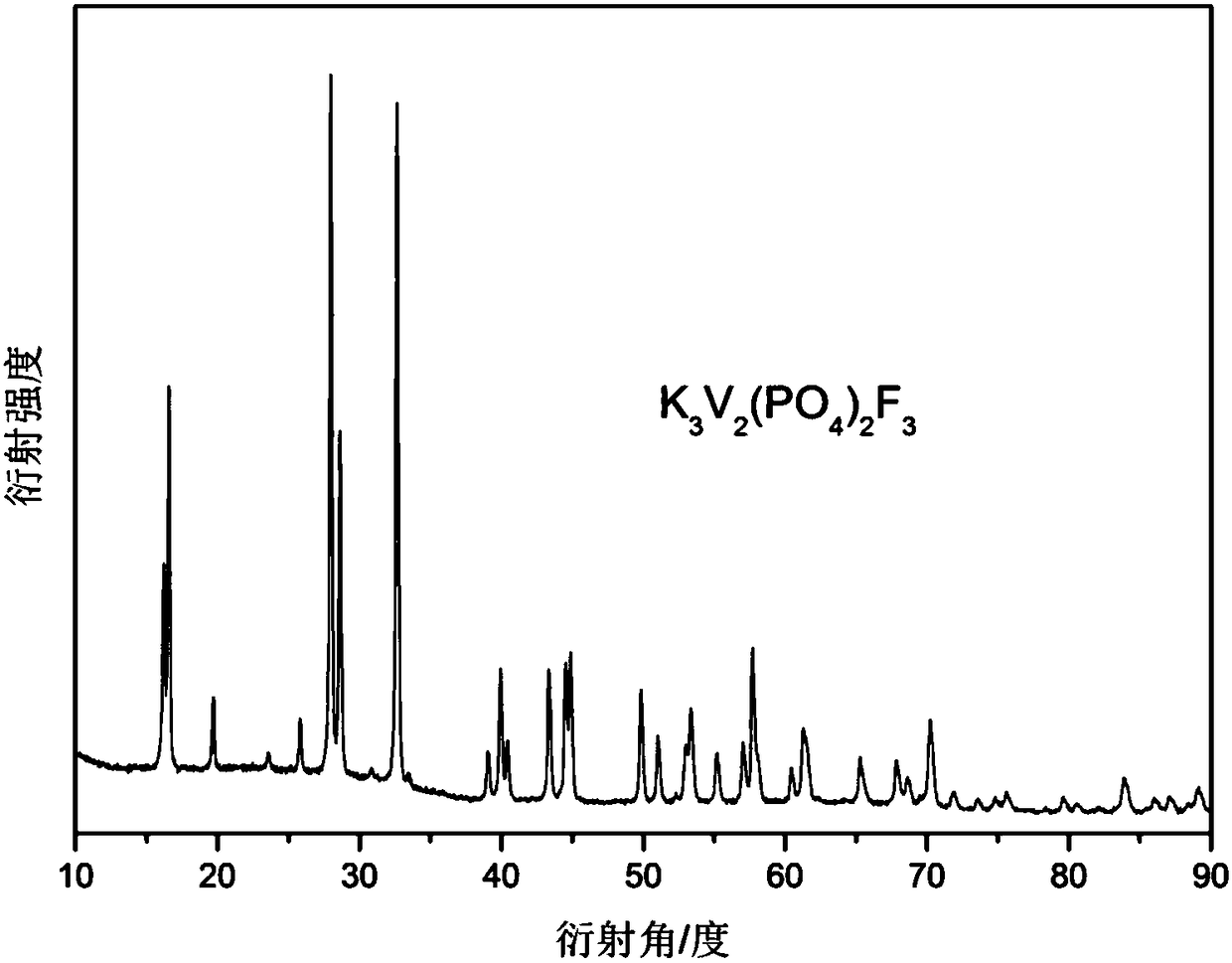
[0019] Implementation case 1: with 0.015mol vanadium pentoxide, 0.03mol ammonium dihydrogen phosphate, 0.045mol potassium fluoride and 0.0075mol sucrose, and all transfer in the ball mill jar, and add the polyoxyethylene glycol of solid mixture weight 20% ( The molecular weight is 200), start the ball mill, mix the mixture evenly, and dry the mixture in an oven at 180°C, transfer the dried mixture to a tube furnace, under the protection of an argon atmosphere, at a rate of 2°C / min The heating rate is heated to 350°C and kept at a constant temperature for 4 hours, then heated to 700°C, kept at a constant temperature for 2 hours, and cooled to room temperature with the furnace to obtain the positive electrode material K for a potassium ion battery. 3 V 2 (PO 4 ) 2 f 3 / C Composite. figure 1 is the X-ray diffraction pattern of the material, figure 2 A scanning electron microscope image of the material.
[0020] The prepared potassium ion cathode material K 3 V 2 (PO4) 2...
Embodiment example 2
[0021] Implementation example 2: 0.015mol vanadium trioxide, 0.03mol diammonium hydrogen phosphate, 0.045mol potassium hydroxide, 0.045mol ammonium fluoride and 0.015mol glucose are all transferred to a ball mill jar, and 15% by weight of the solid mixture is added Polyethylene glycol (molecular weight: 400), start the ball mill, mix the mixture evenly, then place the mixture in an oven at 160°C, dry for 24h, transfer the dried mixture to a tube furnace, and protect it in an argon atmosphere , heated to 400°C at a heating rate of 5°C / min and held at a constant temperature for 6 hours, then heated to 750°C, held at a constant temperature for 6 hours, and cooled to room temperature with the furnace to obtain the positive electrode material K for a potassium ion battery 3 V 2 (PO 4 ) 2 f 3 / C Composite. prepared K 3 V 2 (PO 4 ) 2 f 3 / C Composite rough surface, see image 3 The scanning electron microscope image is shown.
[0022] The prepared potassium ion cathode mat...
Embodiment example 3
[0023] Implementation case 3: 0.015mol vanadium pentoxide, 0.03mol diammonium phosphate, 0.045mol potassium fluoride and 0.0075mol sucrose are all transferred to a ball mill jar, and the polyethylene glycol ( The molecular weight is 400), start the ball mill, mix the mixture evenly, place the mixture in an oven at 180°C, and dry it for 12h, then transfer the dried mixture to a tube furnace, under the protection of an argon atmosphere, at 10°C / Min heating rate to 350°C and constant temperature for 8 hours, then heated to 800°C, constant temperature for 3 hours, and cooled to room temperature with the furnace, that is, the positive electrode material K for potassium ion battery 3 V 2 (PO 4 ) 2 f 3 / C Composite.
[0024] The prepared potassium ion cathode material K 3 V 2 (PO 4 ) 2 f 3 / C composite material, acetylene black and PVDF are ground and mixed evenly in a mass ratio of 8:1:1, and an appropriate amount of NMP is added dropwise to make a slurry, and the slurry i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com