A method for adding oxygen to desulfurize sulfur-containing hydrocarbons
A technology for sulfide oxidation and oxygen supply, which is applied in the field of desulfurization of sulfur-containing hydrocarbons. It can solve the problems of difficult recycling of catalysts, lower octane number of gasoline products, and lower quality of gasoline products, so as to optimize the quality of gasoline products and increase octane number. , the effect of reducing unit consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
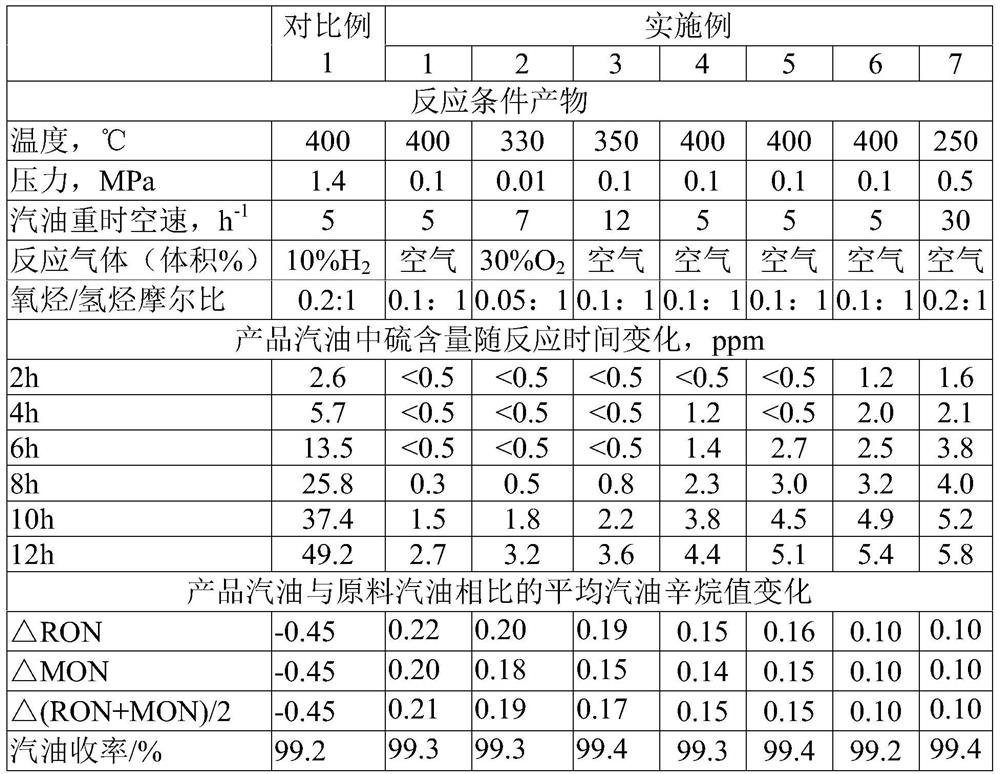
Examples
Embodiment 1
[0050] This example is used to illustrate the method for oxidative desulfurization of sulfur-containing hydrocarbons of the present invention.
[0051] (1) Preparation of catalyst
[0052] 2 kg of kaolin (from Qilu Petrochemical Catalyst Factory, containing 1.6 kg on a dry basis) and 5.1 kg of aluminum sol (commercially purchased from Qilu Petrochemical Catalyst Factory, with an alumina content of 21% by weight) were mixed and stirred for 30 minutes to obtain a carrier slurry. The carrier slurry is spray-dried using a Niro Bowen Nozzle TowerTM type spray dryer, the spray-drying pressure is 8.5 to 9.5 MPa, the inlet temperature is below 500°C, and the outlet temperature is about 150°C. The microspheres (average diameter of 80 μm) obtained by spray drying were first dried at 150° C. for 1 h, and then calcined at 480° C. for 2 h to obtain the carrier.
[0053] 1.5 kg of lanthanum nitrate (commercially purchased from Sinopharm Chemical Reagent Company, analytically pure) was diss...
Embodiment 2
[0060] This example is used to illustrate the method for oxidative desulfurization of sulfur-containing hydrocarbons of the present invention.
[0061] (1) Preparation of catalyst
[0062] 0.37kg of pseudo-boehmite (commercially purchased from Qilu Catalyst Factory, containing 65% by weight of alumina) and 1.23kg of deionized water (pH=7, the same below) were mixed and beaten evenly, and a concentration of 18% by weight of hydrochloric acid was added dropwise , adjusting the pH value of the slurry to 1.8 to make the slurry in a gel state, and aging at 60° C. for 1 hour to obtain peptized pseudo-boehmite.
[0063] With 0.4kg of diatomite (Qilu Petrochemical Catalyst Factory, containing 0.24kg on a dry basis) and 0.794kg of cerium nitrate hexahydrate (Sinopharm Chemical Reagent Company, analytically pure, 0.32kg in terms of cerium oxide) in 2kg of deionized water Mix and stir evenly to obtain rare earth-containing diatomite; add 0.8kg tungsten oxide (analytically pure, containi...
Embodiment 3
[0068] This example is used to illustrate the method for oxidative desulfurization of sulfur-containing hydrocarbons of the present invention.
[0069] (1) Preparation of catalyst
[0070] 0.63kg of SB powder (German Sasol company, containing 69.8% by weight of alumina) and 1.13kg of deionized water (pH=7, the same below) were mixed and beaten evenly, and the hydrochloric acid with a concentration of 18wt% was added dropwise to adjust the pH of the slurry to Adjust to 1.8 to make the slurry in a gel state, and age at 60°C for 1 hour to obtain peptized SB powder.
[0071] With the expanded pearl salt powder of 2.75kg (Beijing Chemical Plant, containing dry basis 2.2kg), the manganese nitrate of 2.46kg (Sinopharm Chemical Reagent Company, analytically pure, with MnO 2 Calculated as 1.24kg) and 0.2kg of neodymium oxide (Sinopharm Chemical Reagent Company, analytically pure) mixed in 7.1kg of deionized water, after stirring for 30min to obtain a mixed slurry containing manganese ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com