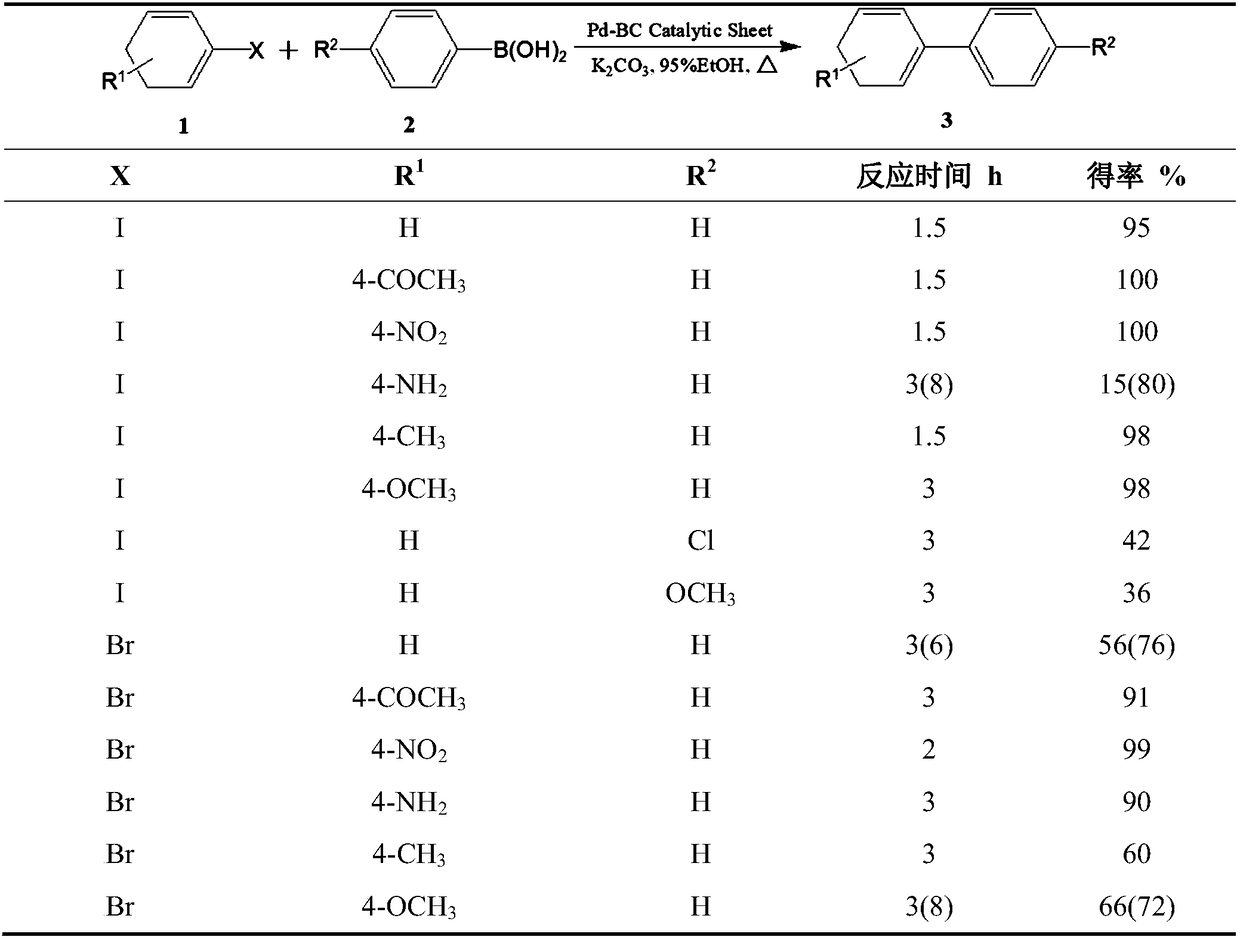
Catalytic test paper produced by compounding bacterial cellulose-supported metal particles with plant fibers, and production method thereof
A bacterial cellulose and plant fiber technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, organic compound/hydride/coordination complex catalyst, carbon-based compound preparation, etc., can solve the problem of weakening reactants and Metal particle contact, weak liquid permeability, poor mechanical properties, etc., to achieve good dispersion and stability, low manufacturing cost, and high reusability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
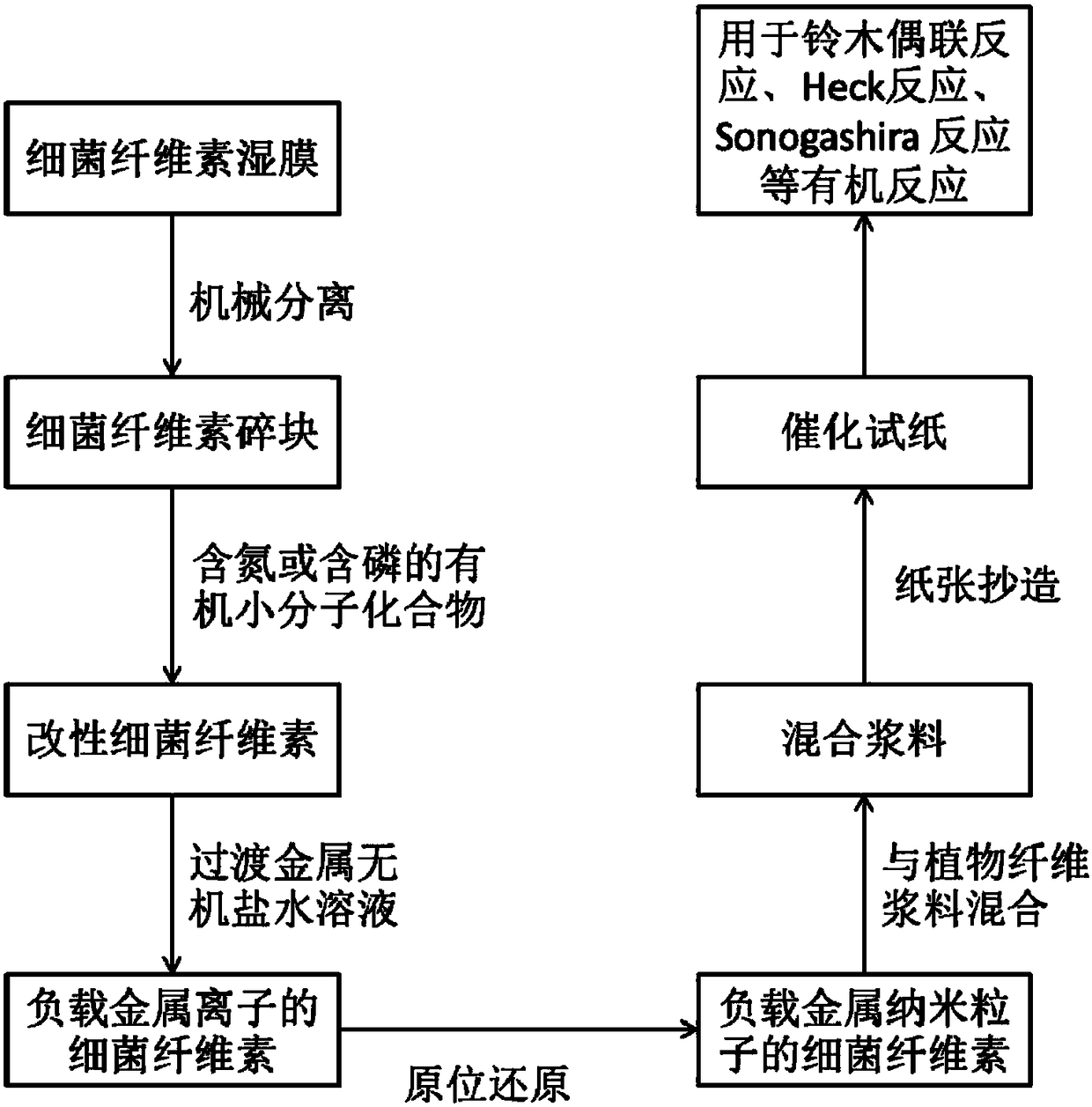
[0032] Cut 30 g of bacterial cellulose (BC) wet film into small pieces, add it to 100 mL of water, and use a tissue grinder to separate it into small pieces (2 mm in each direction), so that it will not be suspended in water after standing for a period of time As standard, after filtering, add to 100mL sodium periodate solution with a mass fraction of 0.2%, and stir at 350rpm. The reaction was carried out at room temperature in the dark for 2 days. After the reaction is complete, the oxidized bacterial cellulose is filtered and washed. Mix oxidized bacterial cellulose with 5.6 g of polyethyleneimine and 80 mL of deionized water into an Erlenmeyer flask. 0.21 g of sodium cyanoborohydride was added as a catalyst. The pH of the mixture was adjusted to 5.8-6 with 0.1M hydrochloric acid. The reaction was performed under magnetic stirring at 350 rpm at room temperature for 6 hours. After the reaction, the polyethyleneimine-modified BC was filtered and washed.
[0033] 0.5 g of ...
Embodiment 2
[0040] Cut 30 g of bacterial cellulose (BC) wet film into small pieces, add 90 mL of deionized water, and use a tissue grinder to separate into small pieces (2mm in each direction), so as not to suspend after standing for a period of time Take water as standard, add 10% mass fraction of sodium hydroxide solution to swell after filtering, and stir at 350rpm for 20min. Then add 15mL epichlorohydrin, filter and wash after reacting for 24h. The epoxidized BC was added to 90 mL of deionized water, 7.6 mL of tetraethylenepentamine and 1.3 g of sodium carbonate were added, reacted at room temperature for 3 hours, and then filtered and washed to obtain tetraethylenepentamine-modified BC.
[0041] 0.5 g of potassium chloropalladate (K 2 PdCl 4 ) was dissolved in 100ml hot water at 80°C, and then tetraethylenepentamine-modified BC was added. The mixture was reacted at 80° C. for 3 hours with magnetic stirring at 350 rpm. The resulting solid product was washed with hot water, added t...
Embodiment 3
[0045] Separate bacterial cellulose (Bacterial Cellulose, BC) wet film 30g into small fragments, the steps are the same as in Example 1. After filtering the BC moisture, add it to 100mL pyridine, heat to 80°C, and stir at 500rpm for 30min. After cooling to room temperature, 10 mL of diphenylphosphine chloride was added and reacted at room temperature for 3 days at a speed of 350 rpm. After the reaction, filter and wash to obtain diphenylphosphine functionalized BC. Diphenylphosphine functionalized BC was added to 100 mL of 0.2 M nickel chloride hexahydrate solution (NiCl 2 .6H 2 O), at room temperature with 350rpm rotating speed stirring reaction 4 hours. The resulting solid product was filtered and washed, and added to 100 mL of 0.1 M sodium cyanoborohydride solution, stirred and reacted at room temperature for 1 hour, and the loaded nickel ions were reduced in situ. The resulting nickel nanoparticles loaded BC (Ni-BC) was filtered and washed with deionized water.
[004...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com