A kind of preparation method of 3-(substituted/non-substituted phenyl)-3-hydroxypropionylhydroxamic acid
A technology of hydroxypropionyl hydroxamic acid and ethyl hydroxypropionate is applied in the field of preparation of aryl propionyl hydroxamic acid compounds, and can solve the problems such as inability to be applied in practical production, expensive ethyl bromoacetate, increased operational complexity, and the like, Achieve the effect of optimizing the synthesis route, improving the total yield and reducing the preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
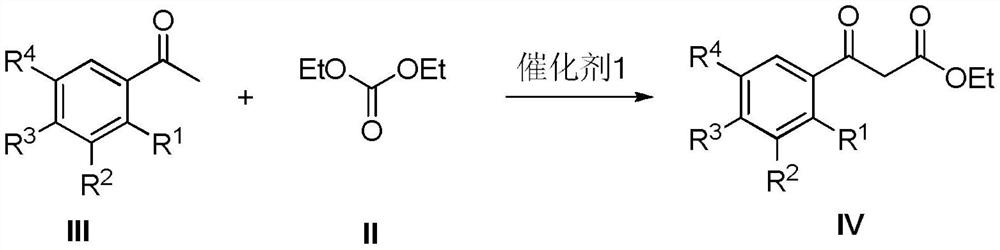
[0049] Embodiment 1: Preparation of intermediate 3-(3-chlorophenyl) ethyl propionate-3-ketone (Ⅳ)
[0050] Dissolve 141.6 grams of diethyl carbonate in 1000 mL of 1:1 ethanol-tetrahydrofuran (THF), add 12.4 grams of catalyst 1 Amberlite IRA-400 under stirring, heat up to 45 °C, and dissolve 154.5 grams of m-chloroacetophenone in 1000 mL of the above ethanol -THF mixed solvent was added dropwise within 1h, continued to stir for 6h, filtered and recovered Amberlite IRA-400, AmberliteIRA-400 was washed 3 times with the above ethanol-THF mixed solvent, the filtrates were combined, most of the solvent was evaporated, and added under stirring 1500mL water, CH 2 Cl 2 Extraction, washed with saturated brine to neutral, anhydrous MgSO 4 Dry, filter, evaporate the solvent, and distill under reduced pressure to obtain 204 g of colorless oily liquid 3-(3-chlorophenyl) ethyl propionate-3-one, with a yield of 90%;
Embodiment 2
[0051] Embodiment 2: by the similar method of embodiment 1, from solvent type, catalyst type and material ratio reaction condition has been studied, research results are shown in Table 1.
[0052] Table 1 yields of compound IV under various reaction conditions.
[0053]
[0054]
[0055]
[0056]
[0057]
[0058]
[0059] Note: Other conditions of the above research: reaction temperature 60°C; reaction time TLC traced until m-chloroacetophenone was reacted or reacted for 48 hours; catalyst dosage was calculated according to m-chloroacetophenone.
[0060] As can be seen from the table:
[0061] 1. For most catalysts, the three solvents of methanol, ethanol and ethanol-THF are beneficial to the reaction. In many cases, the yield can exceed 50%, especially when ethanol-THF is used as the solvent, the yield can reach up to 87% ; Solvents such as ether and water are unfavorable to the reaction.
[0062] 2. As for the catalyst, NaOH and NaOEt, which are commonly...
Embodiment 3
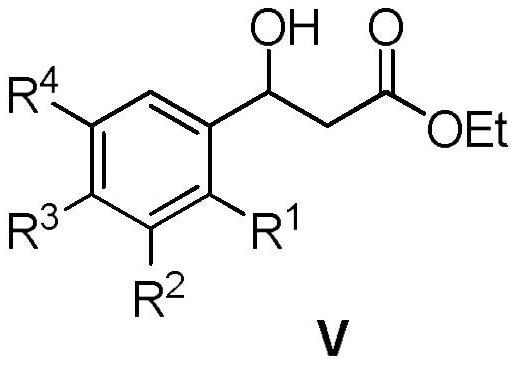
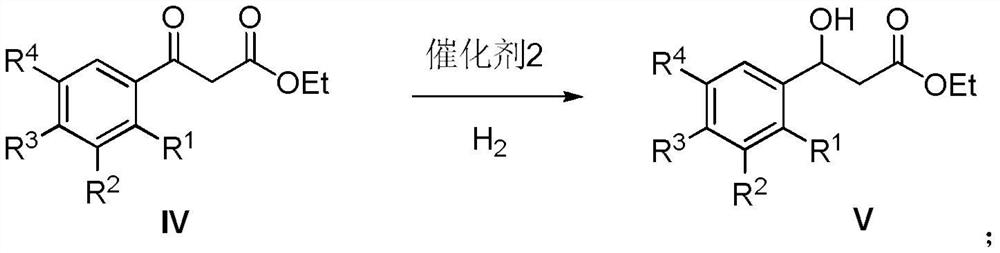
[0065] Embodiment 3: the preparation of intermediate 3-(3-chlorophenyl)-3-hydroxypropionic acid ethyl ester (Ⅴ)
[0066] Dissolve 226.5 g of 3-(3-chlorophenyl) ethyl propionate-3-one in 1500 mL of ethanol, add 80 mL of glacial acetic acid, and feed H under stirring. 2 Remove the air in the reaction system, add 11.3 grams of Pd-C, heat up to 40 ° C, continue to 2 React under the atmosphere for 6 hours, filter, recover the catalyst, and evaporate the solvent under reduced pressure to obtain a light yellow oily substance, ethyl 3-(3-chlorophenyl)-3-hydroxypropionate, with a yield of 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com