Preparation method of core-shell borylated magnetic microspheres capable of enriching glycoproteins in large quantities
A technology of magnetic microspheres and glycoproteins, applied in the preparation of microspheres, microcapsule preparations, etc., can solve the problems of poor control of structure and magnetic content, and achieve the effects of controllable magnetic content, high magnetic response and excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of core-shell iron ferric oxide@silica@polypropyleneamine-carboxyphenyl borate magnetic composite microspheres with a shell thickness of about 25nm and a particle size of about 250nm
[0030] The specific steps are:
[0031] (1) Preparation of magnetic Fe3O4 nanoparticles
[0032] Dissolve 2g of ferric chloride hexahydrate and 4g of sodium acetate in 10mL of ethylene glycol / ethylenediamine (2:1, V / V) mixed solvent, stir mechanically at room temperature for 15 minutes, then transfer the solution into In a tetrafluoroethylene-lined stainless steel high-temperature and high-pressure reaction kettle, place the reaction kettle in an oven at 200°C for 20 hours, and cool to room temperature naturally; use a magnet to separate the black sample, and then wash the black substance with absolute ethanol 4 times , washed with purified water three times, separated the sample with a magnet, and dried in a vacuum oven at 30-50°C for 12 hours. The dried magnetic ...
Embodiment 2
[0041] Example 2 Preparation of core-shell iron ferric oxide@silica@polypropyleneamine-carboxyphenyl borate magnetic composite microspheres with a shell thickness of about 40nm and a particle size of about 300nm
[0042] The specific steps are:
[0043] (1) Preparation of magnetic Fe3O4 nanoparticles
[0044] With embodiment 1.
[0045] (2) Preparation of ferroferric oxide@silicon dioxide nano-magnetic composite particles
[0046] With embodiment 1 step.
[0047] (3) Preparation of vinyl-modified ferric oxide@silica nano-magnetic composite particles
[0048] With embodiment 1.
[0049] (4) Preparation of Fe3O4@Silicon Dioxide@Polypropyleneamine Nanomagnetic Composite Particles
[0050] Wherein the consumption of allylamine is 1mL; Others are the same as in Example 1.
[0051] (5) Preparation of ferroferric oxide@silica@polyacrylamine-carboxyphenyl borate magnetic composite microspheres
[0052] With embodiment 1.
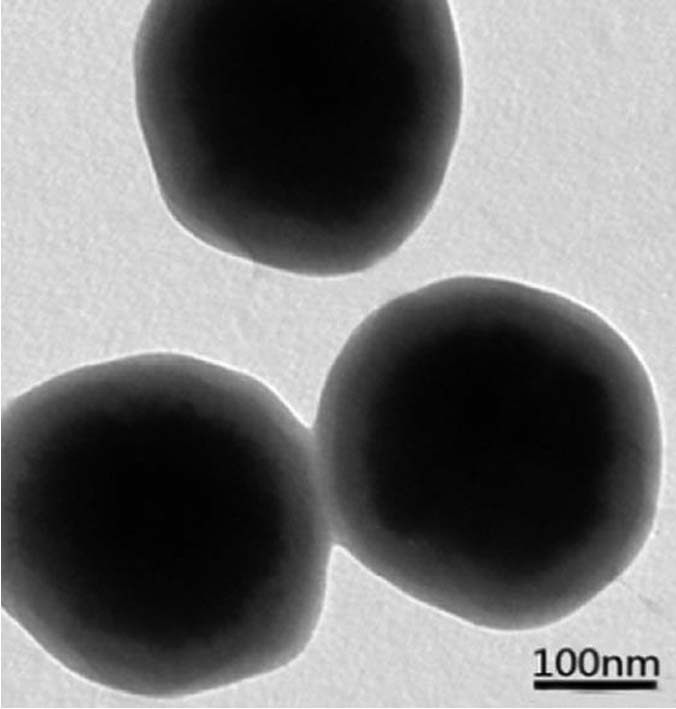
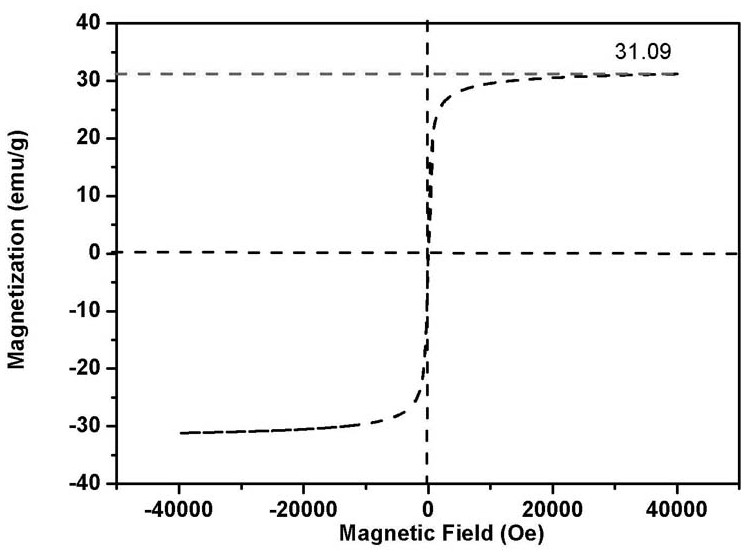
[0053] figure 1 , figure 2 The transmission electro...
Embodiment 3
[0054] Example 3 Using the magnetic composite microspheres prepared in Example 2 to conduct an enrichment experiment on the isolated glycoprotein horseradish peroxidase (HRP) in a complex fetal bovine serum system
[0055] The specific method is:
[0056] (1) First, weigh 1 mg ferric oxide@silica@polyacrylamine-carboxyphenyl borate magnetic composite microspheres, and wash twice with 100 μL buffer solution Ⅰ (10mM PBS, pH=7.4);
[0057] (2), then add 5 μL (40 μL / mL) mixture of glycoprotein and 5 μg glycoprotein HRP, add buffer solution I to 100 μL, incubate at room temperature for 10 minutes;
[0058] (3) Collect the supernatant by magnetic separation, add 100 μL of buffer solution I to wash twice;
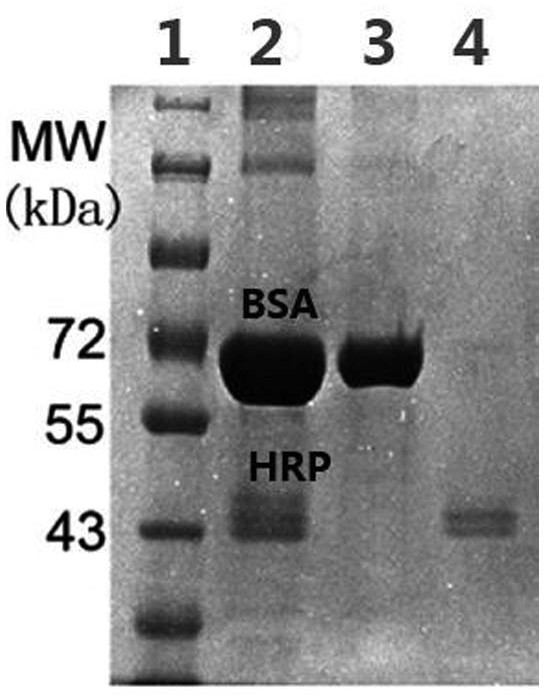
[0059] (4) Finally, elute with 50 μL buffer solution II (50% AN containing 1% TFA), take 10 μL stock solution, 10 μL supernatant and 10 μL eluate, add 10 μL bromophenol blue loading buffer after freeze-drying, and run electrophoresis , the electropherogram of which is shown in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com