Cellulose-polylactic acid amphiphilic drug-loading micelle preparation method and product thereof, and applications of product
A technology of drug-loaded micelles and amphiphilicity, which is applied in the preparation of cellulose-polylactic acid amphiphilic drug-loaded micelles and the preparation of drug-loaded amphiphilic nanomicelles, which can solve the problems of underutilization of renewable resources , to achieve good biodegradability, meet production and application requirements, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
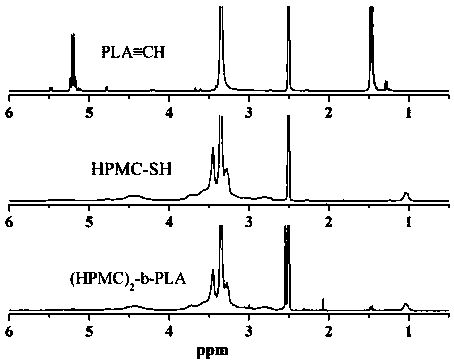
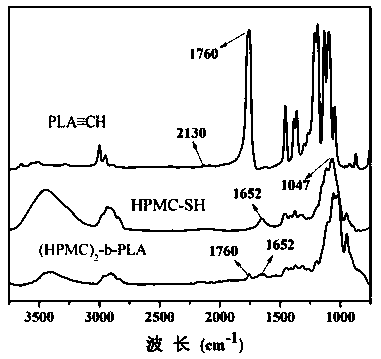
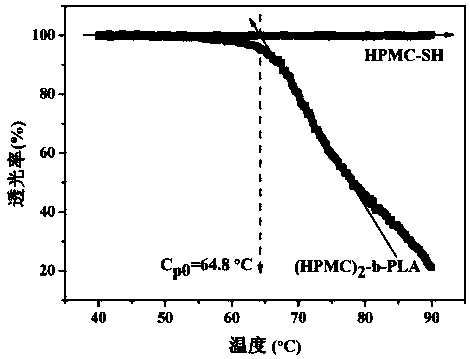
[0034] First, 1 g of hydroxypropylmethylcellulose (Mn=7000 g / mol) and 0.2 g of sodium cyanoborohydride reducing agent were dissolved in 50 mL of dimethyl sulfoxide (DMSO) at 60 °C and 10×10 -3 M NaCl mixed solution (3 / 1 v / v), add 0.1g mercaptoethylamine after complete dissolution, and slowly stir and reflux the above mixed solution at 60 ºC for 6 days, cool the solution to room temperature, and use deionized water in Dialyze in a dialysis bag with a molecular weight cut-off of 3500 g / mol for 3 days, freeze-dry, then add excess dithiothreitol (DTT), react at room temperature for 12 hours under an argon atmosphere, dialyze again for 3 days, and freeze-dry Obtain the hydroxypropyl methylcellulose of end-capping of mercapto;
[0035] Next, 7.2g L-lactide, 168.78 mg propargyl alcohol, 0.2g stannous octoate (Sn(Oct) 2 ) The three substances were added to a Schlenk bottle of 30 mL of anhydrous toluene solution, and the resulting mixture solution was stirred and reacted at 80 ºC for...
Embodiment 2
[0049] First, 1 g of hydroxypropylmethylcellulose (Mn=5000 g / mol) and 0.13 g of sodium cyanoborohydride reducing agent were dissolved in 50 mL of dimethylsulfoxide (DMSO) at 60 °C and 10×10 -3 M NaCl mixed solution (3 / 1 v / v), add 0.15g of mercaptoethylamine after complete dissolution, and slowly stir and reflux the above mixed solution at 60 ºC for 6 days, cool the solution to room temperature, and use deionized water in Dialyze in a dialysis bag with a molecular weight cut-off of 3500 g / mol for 3 days, freeze-dry, then add excess dithiothreitol (DTT), react at room temperature for 12 hours under an argon atmosphere, dialyze again for 3 days, and freeze-dry Obtain the hydroxypropyl methylcellulose of end-capping of mercapto;
[0050] Next, 7.2g L-lactide, 168.78 mg propargyl alcohol, 0.2g stannous octoate (Sn(Oct) 2 ) The three substances were added to a Schlenk bottle of 30 mL of anhydrous toluene solution, and the resulting mixture solution was stirred and reacted at 80 ºC...
Embodiment 3
[0053] First, 1 g of hydroxypropylmethylcellulose (Mn=10000 g / mol) and 0.06 g of sodium cyanoborohydride reducing agent were dissolved in 50 mL of dimethyl sulfoxide (DMSO) at 60 °C and 10 × 10 -3 M NaCl mixed solution (3 / 1 v / v), add 0.08g mercaptoethylamine after complete dissolution, slowly stir and reflux the above mixed solution at 60 ºC for 6 days, cool the solution to room temperature, and use deionized water in Dialyze in a dialysis bag with a molecular weight cut-off of 3500 g / mol for 3 days, freeze-dry, then add excess dithiothreitol (DTT), react at room temperature for 12 hours under an argon atmosphere, dialyze again for 3 days, and freeze-dry Obtain the hydroxypropyl methylcellulose of end-capping of mercapto;
[0054] Next, 7.2g L-lactide, 168.78 mg propargyl alcohol, 0.2g stannous octoate (Sn(Oct) 2 ) The three substances were added to a Schlenk bottle of 30 mL of anhydrous toluene solution, and the resulting mixture solution was stirred and reacted at 80 ºC fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com