Method for directly producing ethyl alcohol by synthesis gas
A synthesis gas and ethanol technology, which is applied in the production of bulk chemicals, chemical instruments and methods, and the preparation of organic compounds, can solve problems such as difficulties in large-scale popularization and application, increased production costs of ethanol, and reduced rhodium usage. The effects of reduced consumption, reduced equipment investment costs, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] Hydrogen form samples were prepared as follows:
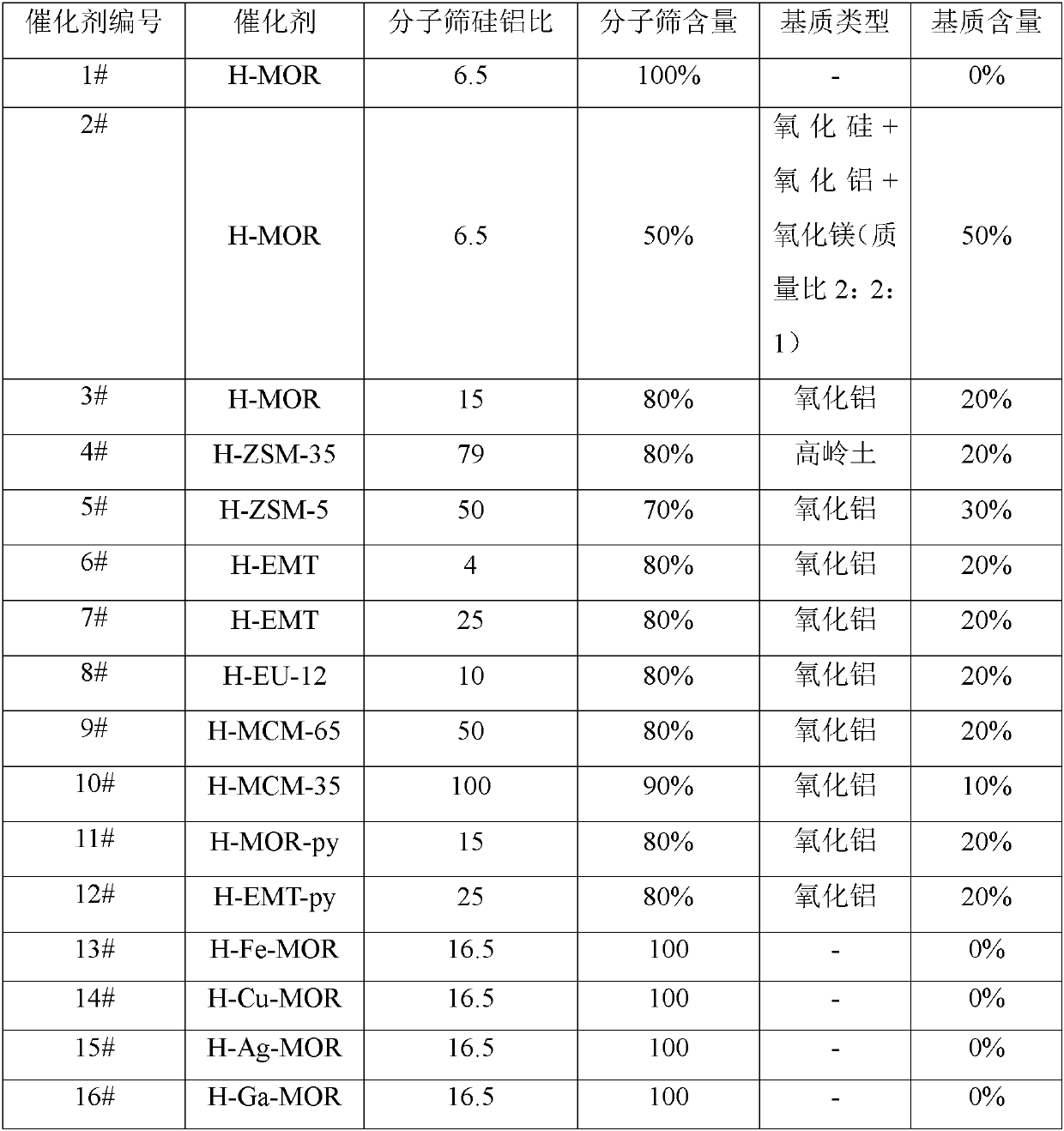
[0054] Pass the Na-type molecular sieves in Table 1 through NH 4 NO 3 Hydrogen molecular sieves are obtained after ion exchange, drying and roasting. For example, the typical hydrogen-form sample preparation process is as follows: In a hydrothermal synthesis kettle, NaMOR molecular sieve powder is added to a pre-configured 1mol / L NH 4 NO 3 In the aqueous solution, the mass ratio of solid to liquid is 1:10, exchange reaction at 80° C. for 2 h under stirring, vacuum filter and wash with water. After three consecutive exchange reactions, drying at 120°C overnight, and calcining at 550°C for 4 hours, the desired catalyst sample HMOR was obtained.
[0055] The matrix-containing molded hydrogen samples were prepared by extrusion molding. For example, a typical sample preparation process is as follows: 80g Na-MOR and 20g alumina are thoroughly mixed, 5-15% nitric acid is added and kneaded, and the kneaded sample is extrude...
Embodiment 1
[0065] Catalyst 11# was used in the first reaction zone, catalyst B (copper-based catalyst) was used in the second reaction zone, and catalyst C was used in the third reaction zone.
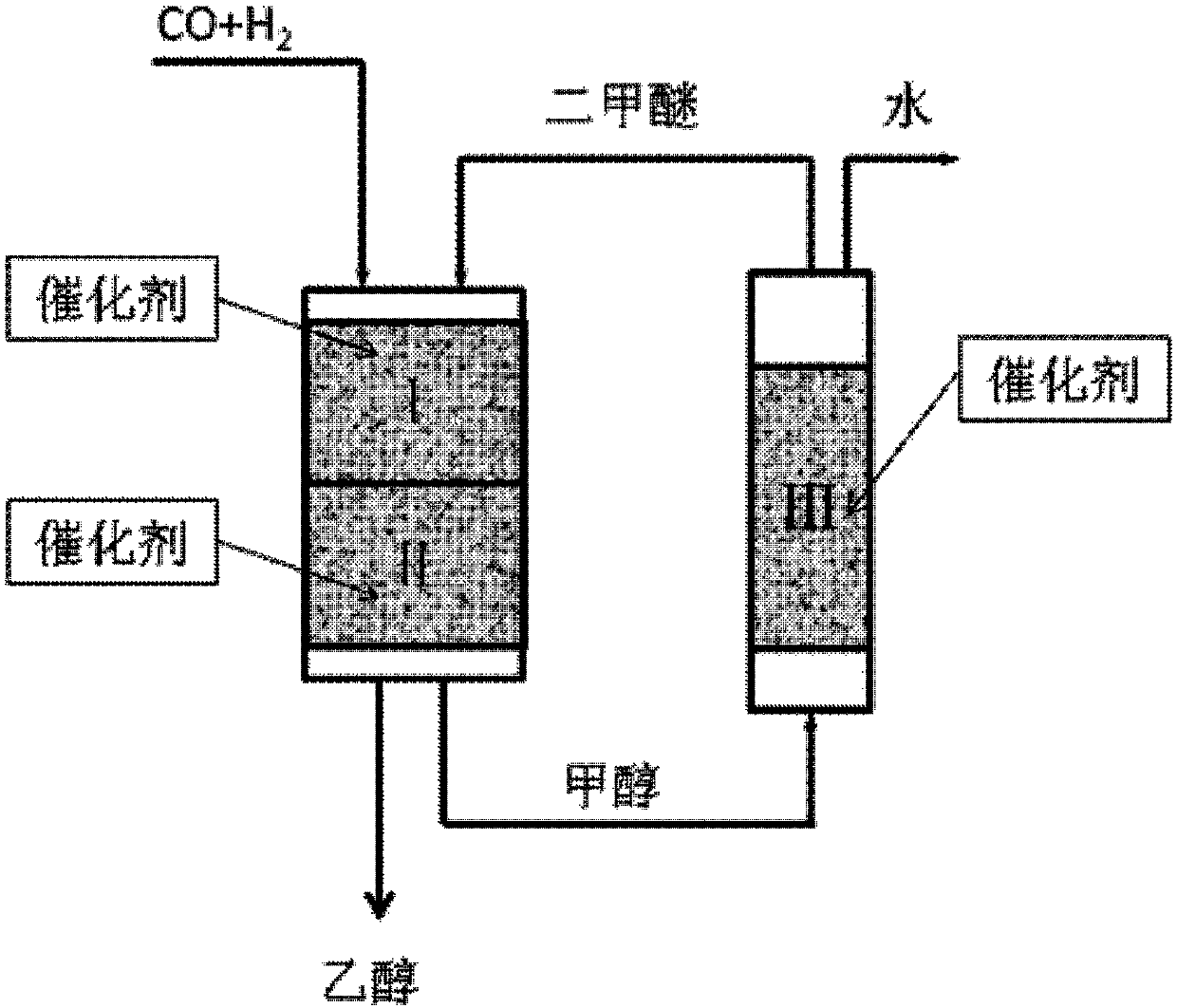
[0066] In a fixed bed reactor, containing CO and H 2 The synthesis gas and dimethyl ether (DME) pass through the first reaction zone and the second reaction zone. The first reaction zone and the second reaction zone are located in the same reactor, wherein the dimethyl ether is completely or partially derived from CO and H 2 Methanol produced in the second reaction zone is dehydrated in the third reaction zone. See the specific reaction process figure 1 , wherein the synthesis gas and dimethyl ether used as raw materials enter the first reaction zone I to contact and react with the solid acid catalyst 11# in the first reaction zone to obtain the effluent containing methyl acetate and / or acetic acid material; the effluent from the first reaction zone enters the second reaction zone II to contact...
Embodiment 2
[0072] The first reaction zone uses different catalysts (1-10# and 12-16#, see Table 4), the second reaction zone uses catalyst B, and the third reaction zone uses catalyst C.
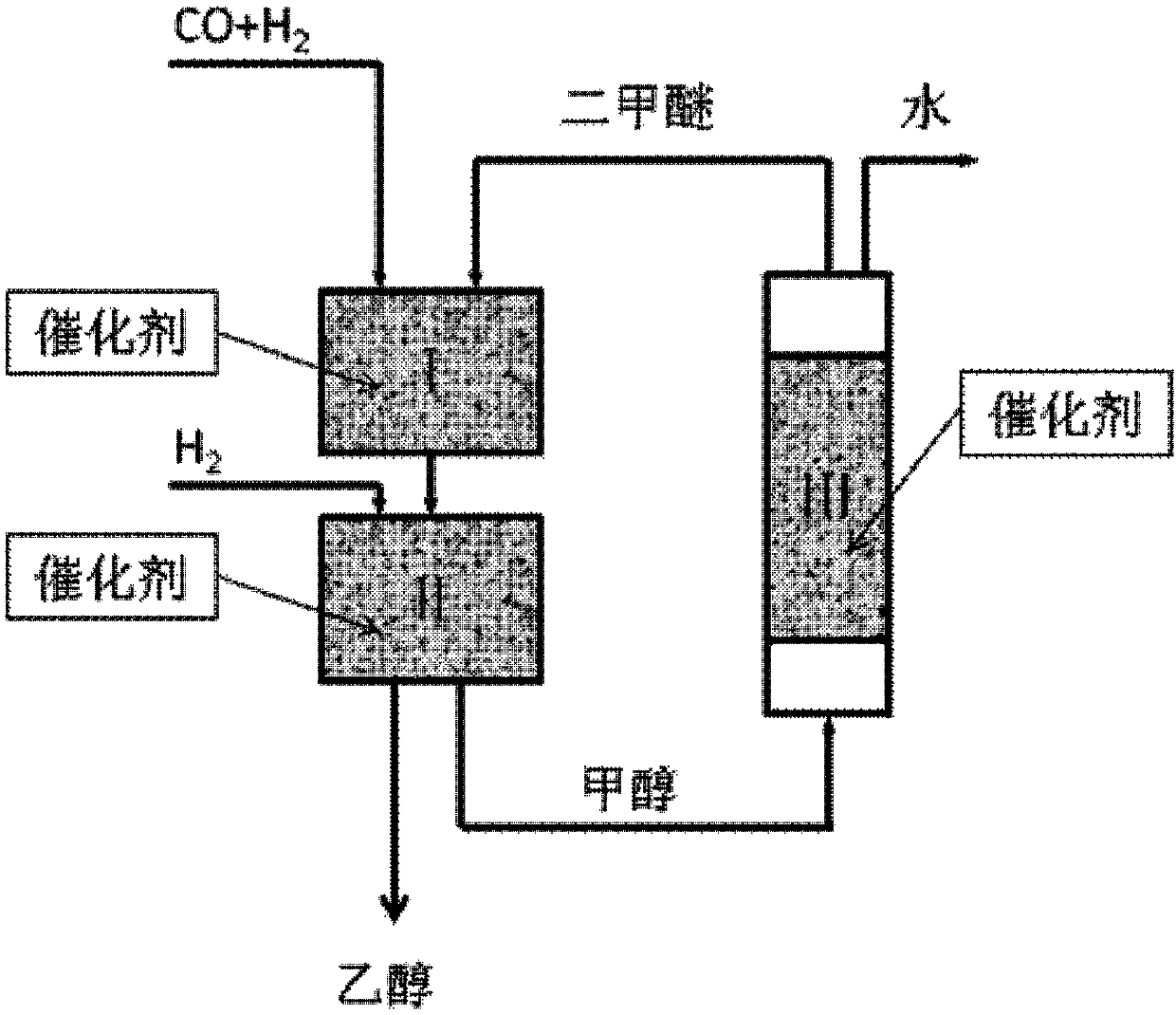
[0073] In a fixed bed reactor, containing CO and H 2 The synthesis gas and dimethyl ether (DME) pass through the first reaction zone and the second reaction zone together, and the first reaction zone and the second reaction zone are located in the same reactor (see the specific reaction process in figure 1 and Example 1), where the dimethyl ether comes from CO and H 2 Methanol produced in the second reaction zone is dehydrated in the third reaction zone. The reaction conditions are as follows: Different catalysts (1-10# and 12-16#, see Table 4) and catalyst B are loaded into the first reaction zone and the second reaction zone of the reactor respectively from top to bottom, and 3g and 7 g; CO, DME and H 2 The molar ratio is 2:1:12; the dimethyl ether feed is 3g / h, the reaction temperature is 215°C, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com