Fluorescent probe for detecting hydrogen sulfide in cells, method for preparing fluorescent probe and application thereof
A fluorescent probe, hydrogen sulfide technology, applied in the field of analytical chemistry, to achieve the effect of cheap raw materials, low preparation cost, and simple and easy synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Synthesis of Fluorescent Probes
[0033] (1) Add 4-ethynylbenzonitrile (1 mmol), 4-bromo-2-hydroxybenzaldehyde (1 mmol), triphenylphosphine (0.02mmol), [1,1-bis(diphenylphosphine Base) Ferrocene] Palladium dichloride (0.02 mmol) was dissolved in triethylamine, after stirring for 10 min, cuprous iodide (0.04 mmol) was added to the reaction system, and heated to reflux at 90°C for 2 hours under the protection of nitrogen , using petroleum ether:ethyl acetate=5:1v / v as the eluent, separated and purified by column chromatography to obtain compound 1: ;
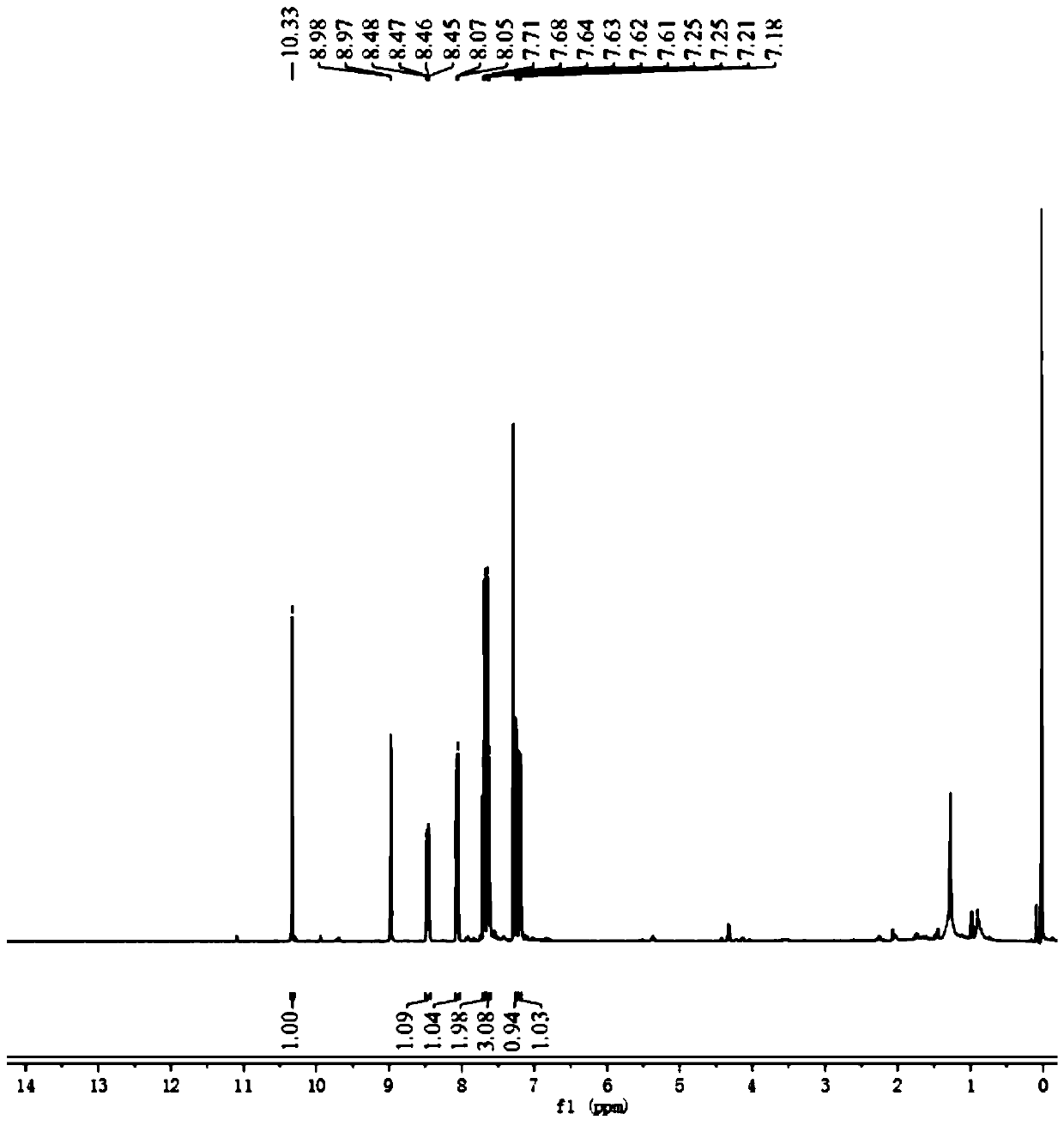
[0034] (2) Dissolve compound 1 (0.3 mmol), 2,4-dinitrofluorobenzene (0.35 mmol) and N,N-diisopropylethylamine (0.3 mmol) in 10 mL of dichloromethane, at room temperature The reaction was stirred for 6 hours, and the target product was obtained by separation and purification by column chromatography with petroleum ether:ethyl acetate=2:1v / v as eluent. 1 H NMR spectrum see figure 1 .
Embodiment 2
[0035] Example 2 Fluorescent probes for different concentrations of Na 2 S's response
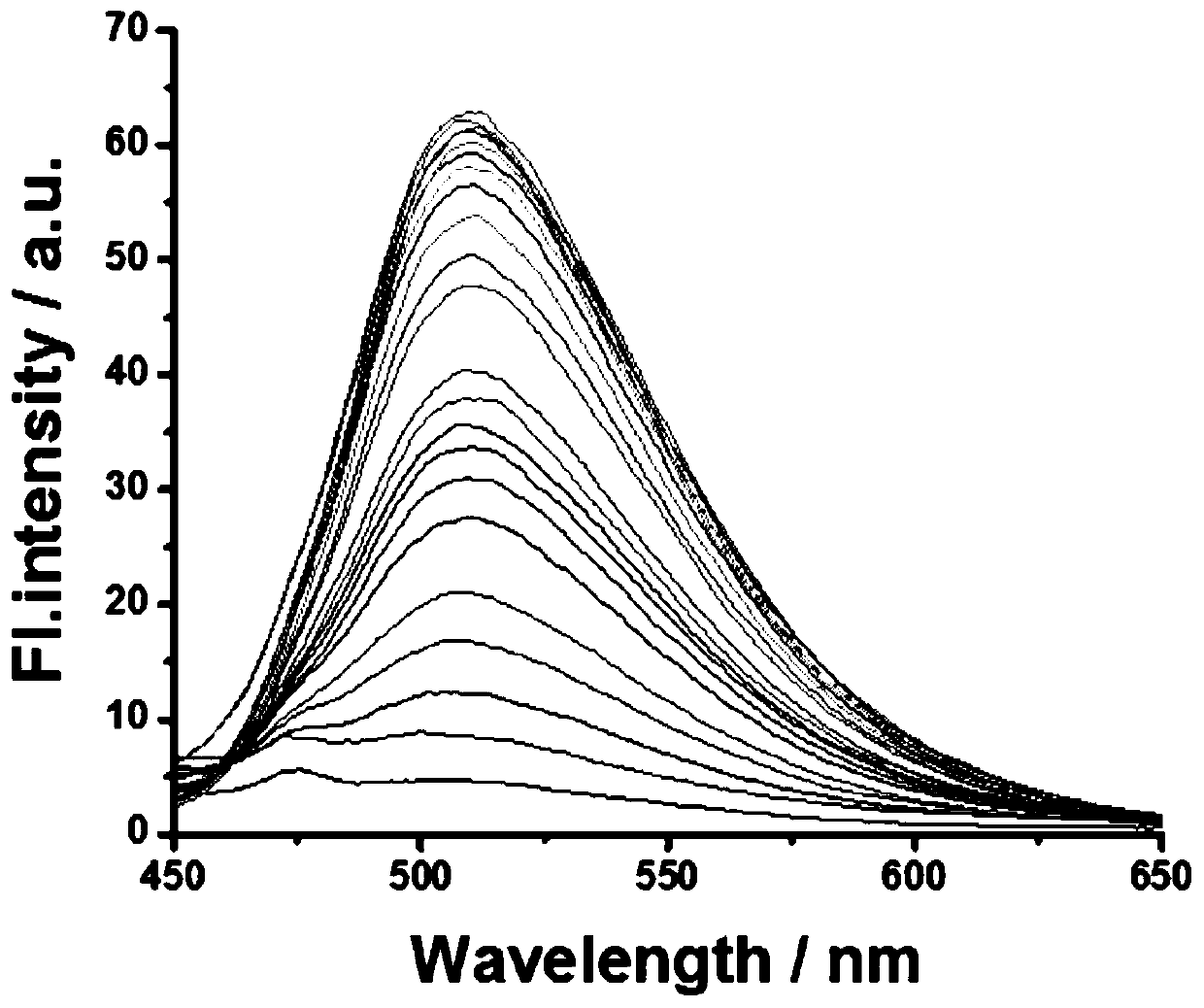
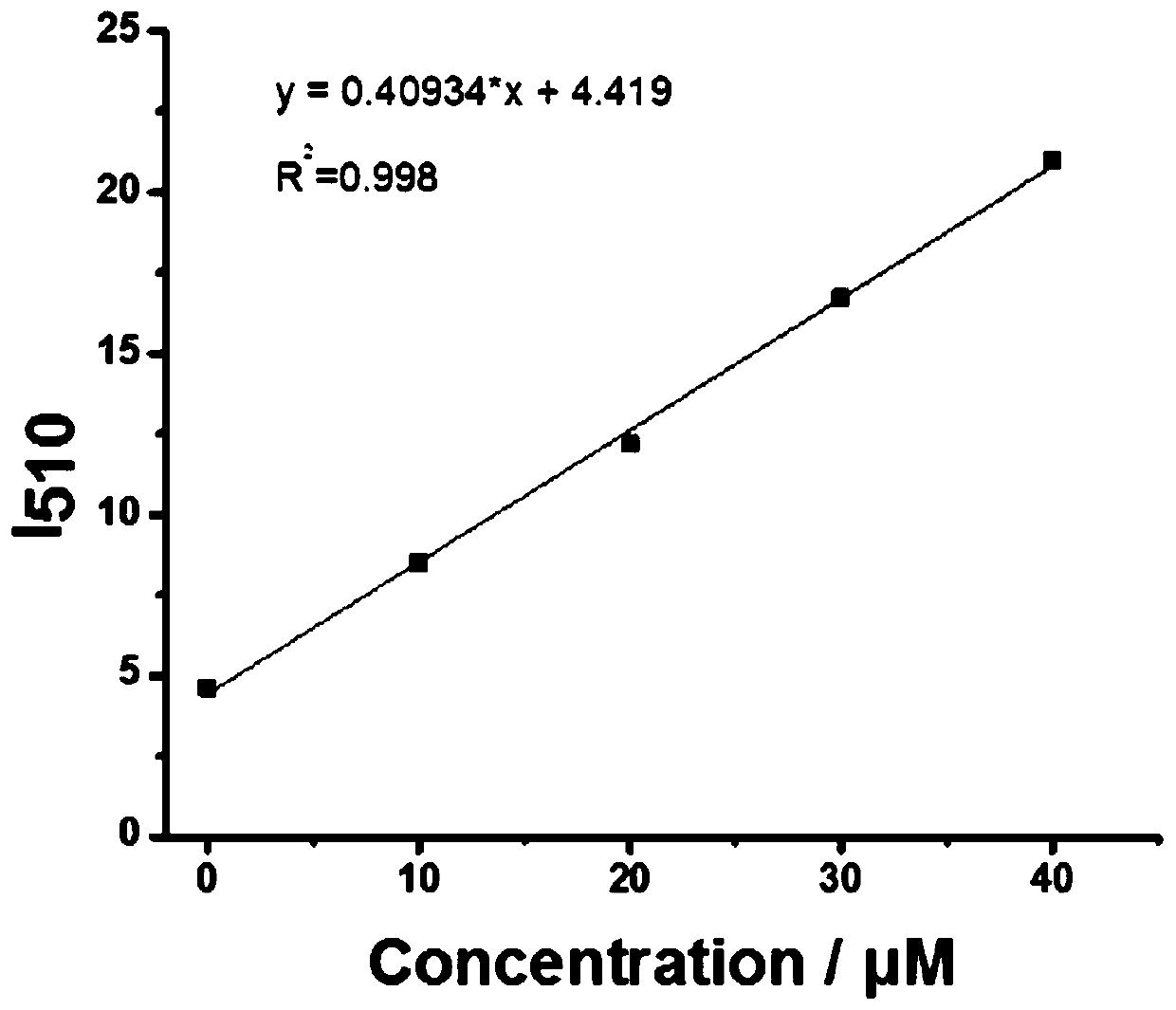
[0036] The probe obtained in Example 1 was dissolved in ethanol, and then diluted with PBS to form a 5 μM probe buffer solution (containing 10% ethanol, pH 7.4). Take 22 parts of the above probe solution, add Na 2 The concentration of S solution is: 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 120, 130, 150, 170, 190, 210, 230, 260, 280, 290, 300 μM, followed by fluorescence detection (λ Ex = 405 nm); calculate the relative fluorescence intensity in each system; the probe has different concentrations of Na 2 S responds as figure 2 Shown: the maximum fluorescence intensity peak is at 510 nm, with Na 2 The fluorescence intensity increased gradually with the increase of S solution concentration. Take Na 2 When the concentration of S is 0, 10, 20, 30, and 40 μM, the concentration of the detected substance is the abscissa, and the corresponding fluorescence intensity at 510 nm (I 510nm ...
Embodiment 3
[0037] Example 3 Selectivity of fluorescent probes to different substances
[0038] The probe obtained in Example 1 was dissolved in ethanol, and then diluted with PBS to form a 5 μM probe buffer solution (containing 10% ethanol, pH 7.4). Take 22 parts of the above probe solution with a volume of 4 mL, add 20 μL of PBS solutions of different substances with a concentration of 40 mM, and then perform fluorescence scanning (λ Ex = 405 nm); calculate the relative fluorescence intensity in each system; take the corresponding fluorescence intensity at 510 nm (I 510nm ) is the ordinate, and the histogram of the response of the probe to different substances is obtained, such as Figure 4 Shown, among them, 1-22 are respectively blank, Al 3+ 、 Ba 2+ , Ca 2+ 、Co 2+ 、Cu 2+ , Cys, F - , glucose, GSH, H 2 o 2 , HClO, Hcy, I - , Mg 2+ , MnO 2 、Ni 2+ , Sn 2+ , SO 3 2- , VC, Zn 2+ 、Na 2 S. It can be seen that the fluorescent probe is only sensitive to the addition of Na ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com