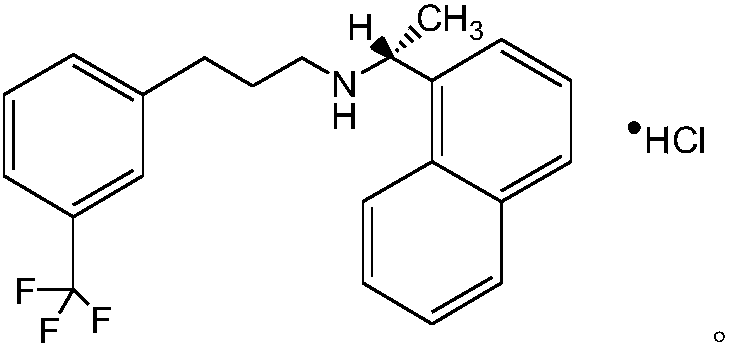
Cinacalcet hydrochloride preparation method
A technology of cinacalcet hydrochloride and dilute hydrochloric acid, which is applied in the field of organic synthesis route design and the preparation of raw materials and intermediates, can solve the problems of difficult post-processing, complicated process operation, incomplete reaction, etc., and promote economic and technological development , The process is simple and the effect of industrialization is realized
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
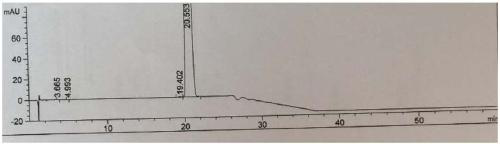
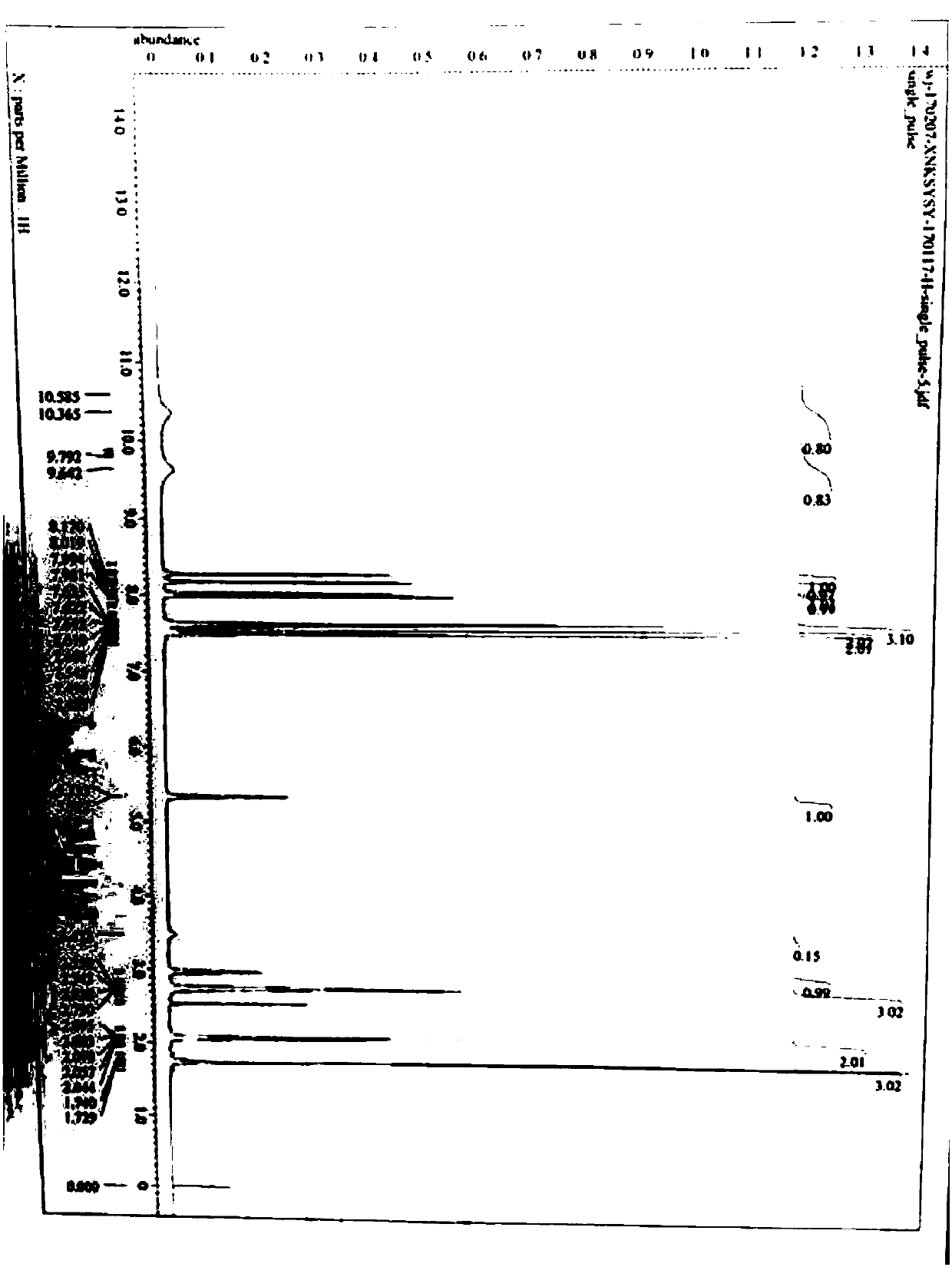
Image
Examples
Embodiment 1
[0059] Embodiment 1: the preparation of sulfonation reaction intermediate I
[0060]
[0061] Put 300g of 3-(3-trifluoromethylphenyl)propanol, 1800ml of toluene, and 246ml of triethylamine into a 5L three-neck flask, protect it with inert gas, stir, and cool the system down to -5~0°C. Control the temperature from -5 to 0°C, and add 127.7ml of MsCl dropwise. After about 2 hours of dripping, keep stirring at -5~0°C for 1 hour, raise the temperature of the reaction system to 20~30°C for 3 hours, and monitor the reaction by TLC (developing agent: petroleum ether: ethyl acetate = 1:1 (volume ratio)) completely. Cool the system down to 5-10°C, control the system temperature to ≤20°C, add 740ml of water, stir, the solids in the system dissolve, separate the liquids, add 250ml of 0.2mol / L HCl to the organic phase for extraction, and then use 740ml of HCl to extract the organic phase 2 O×3 washing (removal of triethylamine hydrochloride), separation of the organic phase, (bath tem...
Embodiment 2
[0062] Embodiment 2: the preparation of cinacalcet base
[0063]
[0064] Add 425g of Intermediate I, 269.4g of (R)-(+)-1-(1-naphthyl)ethylamine, 395.3g of potassium carbonate, and 3L of acetonitrile into a 5L three-necked flask, stir, and heat to reflux (80-83°C ), reflux for 16 to 17 hours, lower the temperature to 65 to 70°C, add 197g of potassium carbonate to the reaction system, heat to reflux, continue to stir and react for 7 to 8 hours, lower the temperature of the reaction system to 25±5°C, filter, and filter the cake Wash with 2L of acetonitrile, combine the filtrate, concentrate the filtrate to dryness under reduced pressure, add 2075ml of dichloromethane to the residue, stir to dissolve completely, then extract with 1200ml of 5% HCl, and use 2×1200ml NaHCO for the organic phase in turn 3 The saturated solution was washed with 1200ml of water, and the organic phase was concentrated to dryness under reduced pressure to obtain 493g of cinacalcet base, with a yield o...
Embodiment 3
[0065] Embodiment 3: Salt-forming reaction, preparation of cinacalcet hydrochloride crude product
[0066]
[0067] Add 493g of cinacalcet base and 493ml of acetonitrile into a 5L three-necked flask, stir to dissolve, and add 4930ml of 1mol / L HCl dropwise at a temperature of 55±5°C. The dropwise addition is completed in about 30 minutes, and a large amount of solids gradually precipitate out during the process. Continue to stir at room temperature (25±5°C) to form salt for 2 hours, lower the temperature of the system to 0-10°C, crystallize for 1-2 hours, filter, wash the filter cake with water, and dry it in a blast oven at 50°C to obtain 503.8g of carbamide hydrochloride. The crude product of Nakasai has a yield of 92.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com