Escherichia coli for high expression of foreign protein and construction method and application thereof
A technology for Escherichia coli and exogenous protein, applied in the field of genetic engineering, can solve the problems of long stable period, difficult to maintain, and low yield of secreted exogenous protein.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] 1. Transformation of BAT47
[0062] Escherichia coli BAT47 (E.coli strain W3110) was purchased from ATCC, and its genotype is: tonA ptr3△phoA△E15△(argF-lac)169degP41△ompT kan R .
[0063] For BAT47(tonA ptr3△phoA△E15△(argF-lac)169degP41△ompT kan R ) to carry out the transformation of spr and ilvG genes, the steps are as follows:
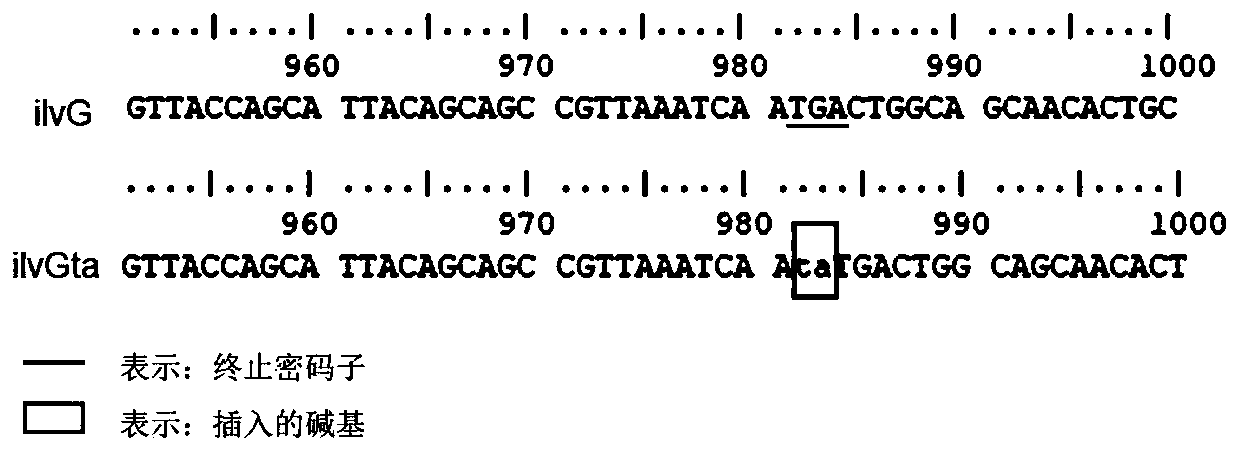
[0064] Step 1: mutate tryptophan (W, Trp) at position 174 encoded by the spr gene of the host bacterium into arginine (R, Arg) (the 520th bp T of the spr gene sequence is mutated into C).
[0065] 1. Construction of targeting vector pCVD442-spr
[0066] Primers were designed with reference to the spr gene (NCBI Locus tag: YP75_p2138) and its upstream and downstream sequences. Using BAT47 genomic DNA as a template, use spr-5F, spr-5R, spr-3F, and spr-3R primers (SEQ ID NO.1-4) with high-fidelity PCR enzymes to obtain full-length spr targeting fragments. Already contains T / C mutation, which can make tryptophan at position 174 of spr gene mu...
Embodiment 2
[0129] Example 2 Design and construction of foreign protein vector expressed in periplasmic space
[0130] The phoA promoter includes two parts, the core promoter (-40bp to +40bp) and the signal peptide. The schematic diagram of the phoA promoter structure is shown in Figure 10 . In order to effectively terminate the process of transcribing the exogenous protein sequence into RNA, a natural terminator such as T7 terminator or rrnB terminator can be used in the selection of the transcription terminator, but in order to make the transcription more effective termination, in this embodiment The engineered terminator λt0 was used (see Sequence No.). In order to enable the foreign protein to be correctly folded in the periplasmic space of E. coli, the sequence encoding the phoA promoter (SEQ ID NO.29) was fused with the foreign protein sequence and the transcription terminator λt0 (the structure diagram of the foreign protein is shown in Figure 11 shown), so that when the protei...
Embodiment 3
[0144] Example 3 Expression of Monoclonal Antibody Fragment Fab
[0145]In a 10L fermenter, the medium components are: casein hydrolysate (casein hydrolysate) 12g / L, sodium citrate dihydrate 1.2g / L, ammonium sulfate 6.2g / L, dipotassium hydrogen phosphate trihydrate 3.5g / L , sodium dihydrogen phosphate dihydrate 1.3g / L, defoamer 0.2ml / L (V / V), magnesium sulfate 1.44g / L, glucose monohydrate 3.36g / L, isoleucine 0.3g / L, three Ferric chloride hydrate 0.0337g / L, zinc sulfate heptahydrate 0.00575g / L, copper sulfate 0.00319g / L, boric acid 0.00124g / L, cobalt chloride hexahydrate 0.00476g / L, manganese sulfate monohydrate 0.00338g / L, Sodium molybdate dihydrate 0.00284g / L, kanamycin 0.05g / L, ampicillin 0.05g / L. Pick a single colony from the streaked LB plate, inoculate it into a 50ml centrifuge tube containing 12ml of LB medium (50μg / ml kanamycin, 50μg / ml ampicillin), and culture overnight at 37°C with shaking at 220rpm. About 19 hours. On the second day, take 10ml of the bacterial liq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com