A kind of four-arm star block polycarboxylic acid superplasticizer and its preparation method and application
A superplasticizer, four-arm star technology, which is applied in the field of four-arm star block polycarboxylic acid superplasticizer and its preparation, can solve the problem of limited controllability of molecular structure, high water reduction rate and low solubility and other problems, to achieve the effects of excellent molecular structure controllability, low water absorption and corrosion, and strong regional adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
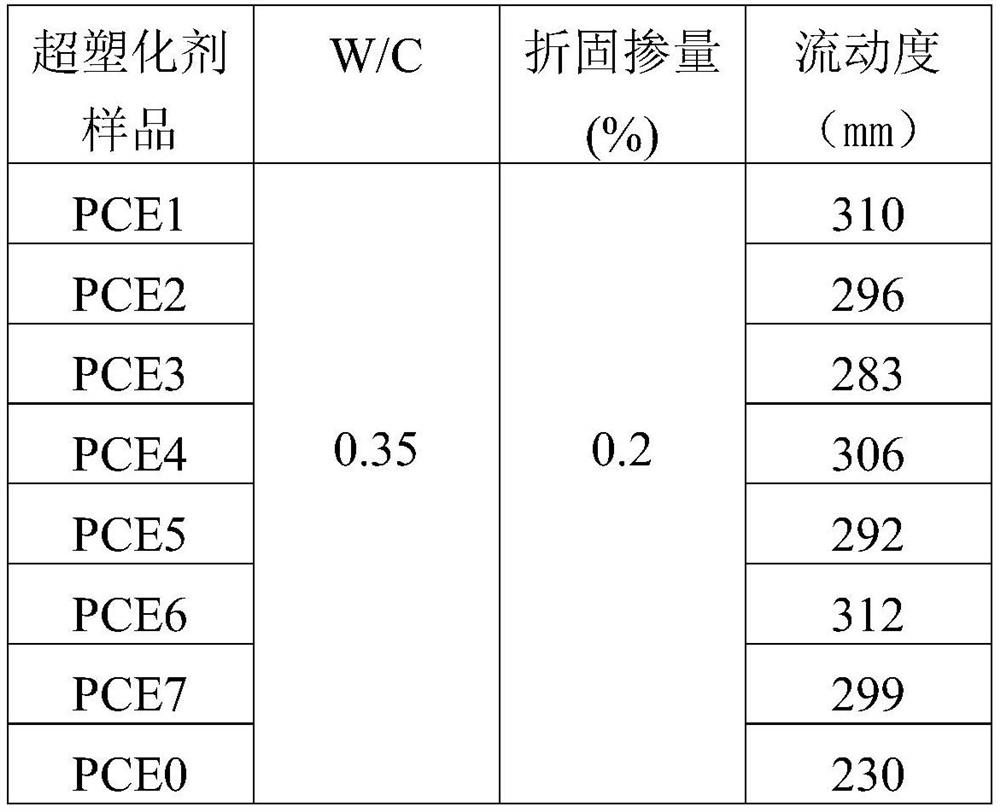
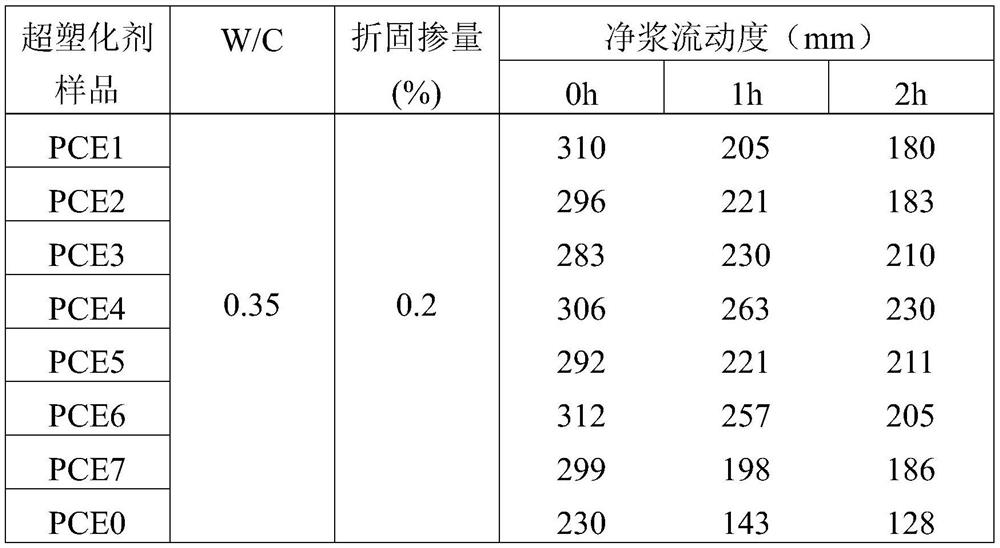
Image
Examples
Embodiment 1
[0047] A preparation method of a four-arm star-shaped block polycarboxylic acid superplasticizer, comprising the following steps:
[0048] (1) 13.6 g of pentaerythritol, 0.1 g of 4-dimethylaminopyridine, and 2-bromoisobutyryl bromide were stirred in a flask at room temperature for 24 hours.
[0049] (2) Add 0.7 g of the product in step (1) to 35 ml of acetone, stir to dissolve, then add 40 g of benzyl methacrylate, 0.144 g of cuprous bromide, 0.144 g of pentamethyldiethylenetriamine, and blow nitrogen gas, Freeze-pump-puff nitrogen, react at room temperature for 3h, and precipitate with methanol:water=1:1 to obtain a macromolecular initiator.
[0050] (3) 0.1g of macromolecular initiator in step (2), 10g of polyethylene glycol methacrylate, 0.144g of cuprous bromide, 0.144g of tetramethylethylenediamine, 20ml of acetone, blow nitrogen, freeze-pump Gas-bubble nitrogen, react at room temperature for 12 h, methanol:N,N-dimethylformamide=1:1 for precipitation.
[0051] (4) Add 0...
Embodiment 2
[0053] A preparation method of a four-arm star-shaped block polycarboxylic acid superplasticizer, comprising the following steps:
[0054] (1) 13.6 g of pentaerythritol, 0.07 g of triethylamine, and 2-bromoisobutyryl bromide were stirred in a flask at room temperature for 24 hours.
[0055] (2) Add 0.7 g of the product in step (1) to 30 ml of tetrahydrofuran, stir to dissolve, then add 56 g of benzyl methacrylate, 0.144 g of cuprous bromide, and 0.144 g of pentamethyldiethylenetriamine, and blow nitrogen. Freezing-pumping-blowing nitrogen, reacting at room temperature for 3h, acetone:water=3:1 for precipitation, to obtain a macromolecular initiator.
[0056] (3) step (2) macromolecular initiator 0.1g, polyethylene glycol acrylate 10g, cuprous bromide 0.144g, bipyridine 0.144g, 20ml of acetone, nitrogen gas, freezing-pumping-nitrogen gas, normal temperature Reaction for 12h, precipitation in ethanol.
[0057] (4) Add 0.1 g of the product in step (3) to 20 ml of tetrahydrofura...
Embodiment 3
[0059] A preparation method of a four-arm star-shaped block polycarboxylic acid superplasticizer, comprising the following steps:
[0060] (1) 13.6 g of pentaerythritol, 0.1 g of pentamethyldiethylenetriamine, and 2-bromoisobutyryl bromide were stirred in a flask at room temperature for 13 hours.
[0061] (2) 36ml of butanone was added to 0.7g of the product in step (1), stirred and dissolved, and then 20g of benzyl methacrylate, 10g of tert-butyl methacrylate, 0.144g of cuprous bromide, and pentamethyldiethylene were added. 0.144 g of base triamine, nitrogen sparging, freezing-pumping-nitrogen sparging, reaction at room temperature for 6 h, methanol:water=5:1 precipitation, to obtain a macromolecular initiator.
[0062] (3) 0.1g of macromolecular initiator in step (2), 10g of polyethylene glycol methacrylate, 0.144g of cuprous bromide, 0.144g of pentamethyldiethylenetriamine, 20ml of ethanol, nitrogen sparging, Freeze-exhaust-bubble nitrogen, react at room temperature for 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com