Carboxylic ester structure containing diene monomer and polyester thereof, and sulfur-containing polyester
A sulfur-containing polyester and carboxylate technology, which is applied in the field of carboxylate-containing diene monomers and their polyesters and sulfur-containing polyesters, can solve the problems of non-renewable, limited fossil resources, and environmental pollution of fossil resources. , to achieve the effect of easy production, simple synthesis process and reduced dependence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] p-Hydroxybenzaldehyde allylation reaction, the reaction equation is as follows:
[0081]
[0082] Experimental steps:
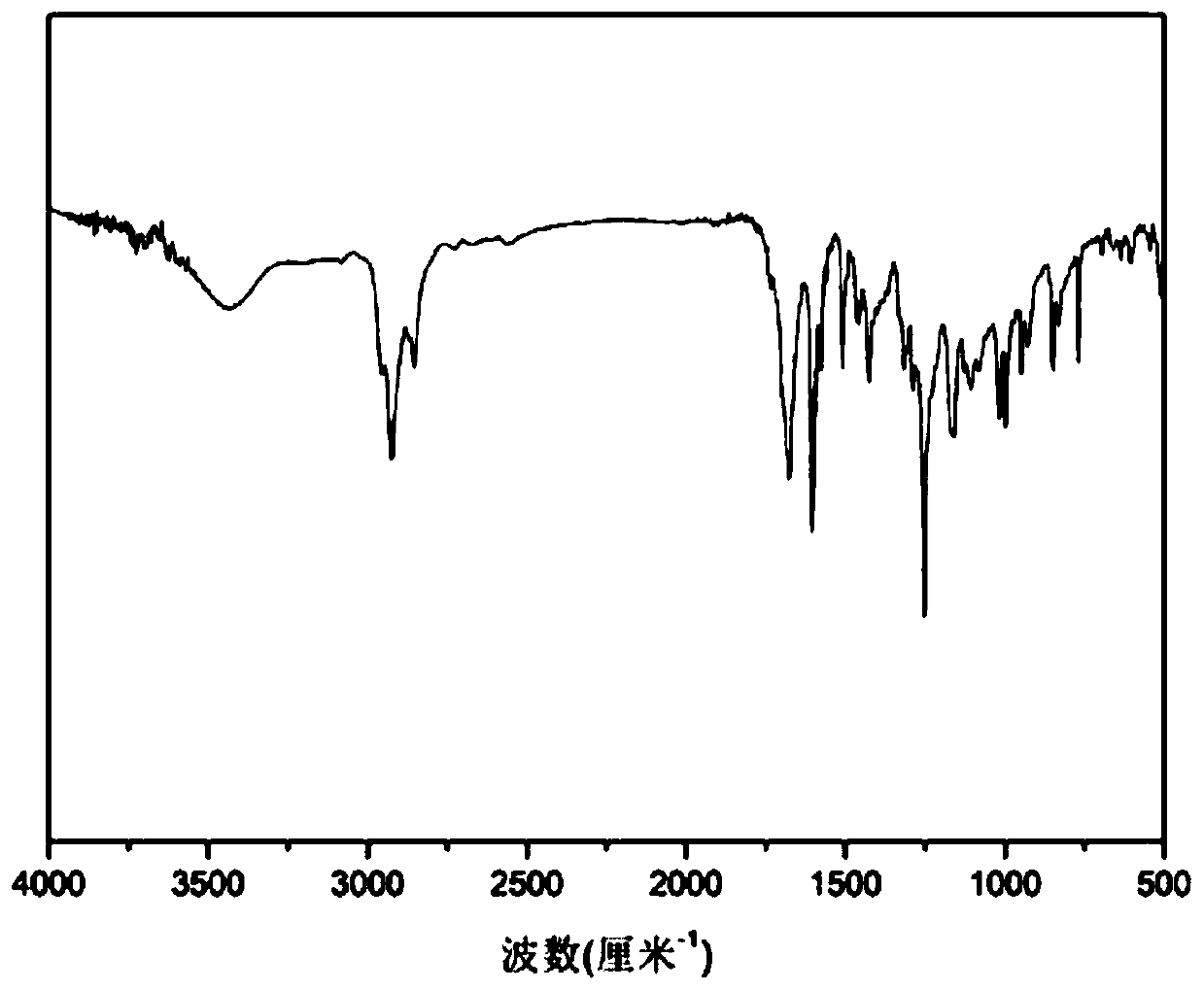
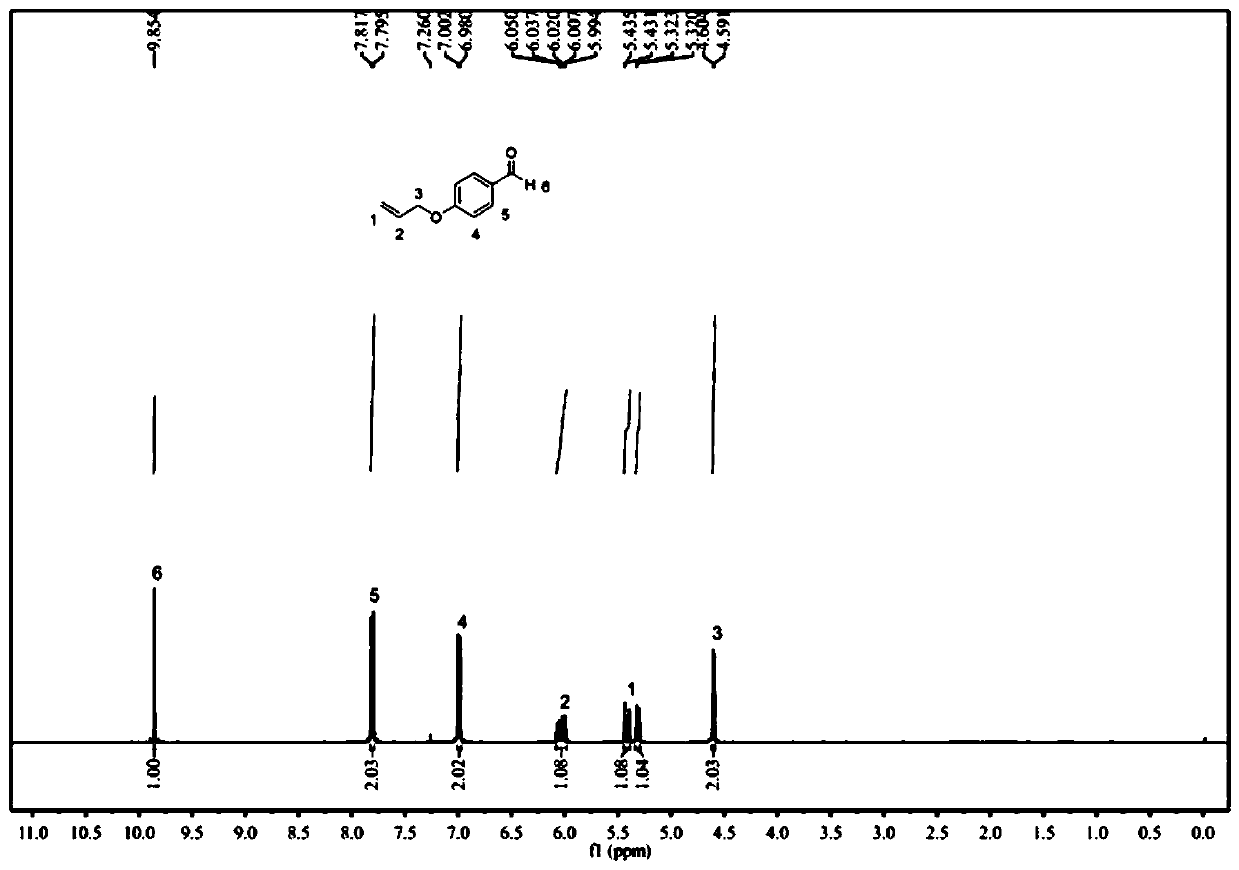
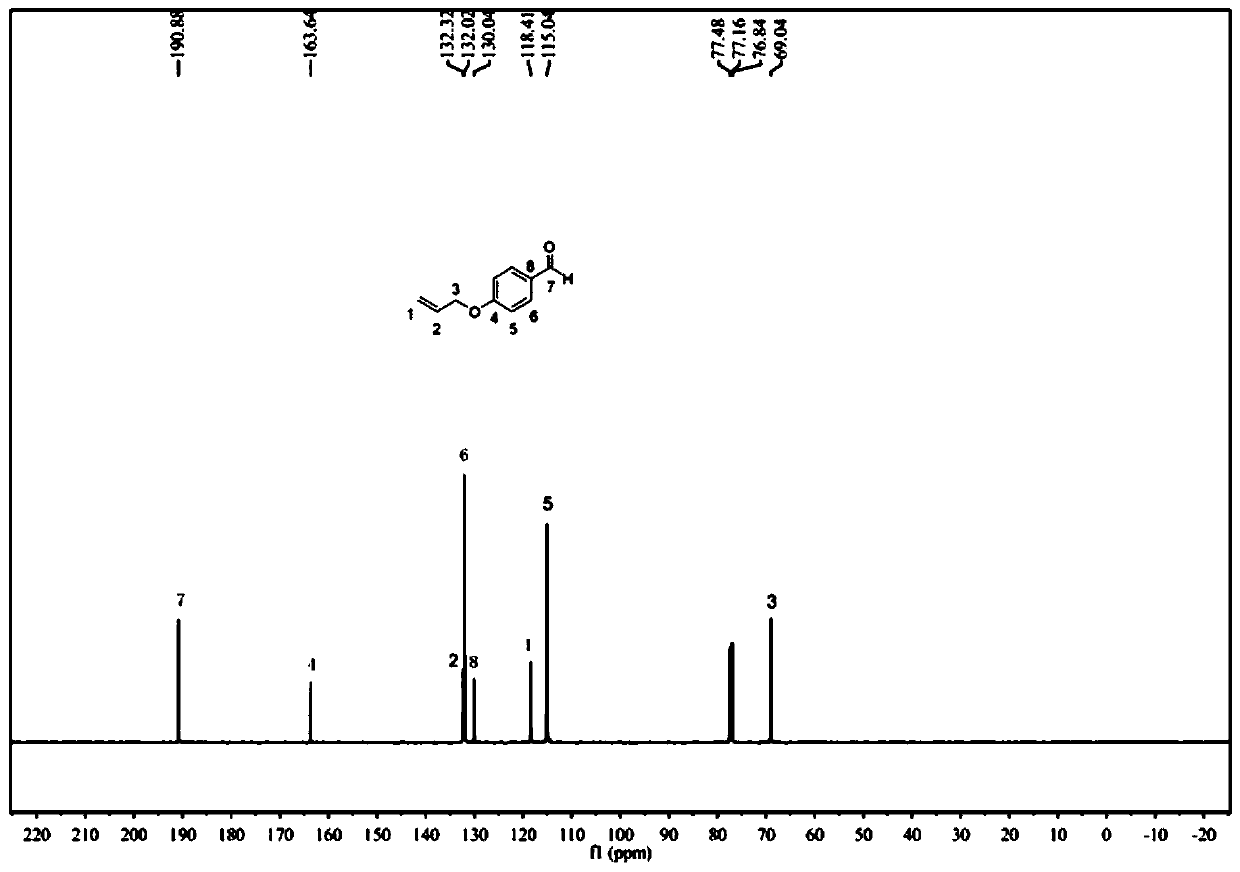
[0083] Add p-Hydroxybenzaldehyde (100 mmol) to the flask and dissolve it in 50 ml of absolute ethanol, add anhydrous potassium carbonate (150 mmol) and stir at room temperature for 30 min, add bromopropene (120 mmol) dropwise under nitrogen atmosphere ), the molar ratio of p-hydroxybenzaldehyde, bromopropene and anhydrous potassium carbonate is 1:1.2:1.5, after the dropwise addition, gradually warming up to 80°C, condensing and refluxing, reacting for 72h, filtering, and removing the solvent under reduced pressure distillation , diluted with a small amount of water, extracted with ethyl acetate and saturated brine. Combine the organic phases, add anhydrous sodium sulfate, let stand overnight, collect by silica gel dry method, use the mixed solvent of petroleum ether / ethyl acetate (volume ratio 6:1) as the eluent for chromatographic column separatio...
Embodiment 2
[0085] Vanillin monomer allylation reaction, the reaction equation is as follows:
[0086]
[0087] Experimental steps:
[0088] Add vanillin (150 mmol) and dissolve in 80 milliliters of absolute ethanol in the flask, add anhydrous potassium carbonate (750 mmol) and stir at room temperature for 30 min, add bromopropene (180 mmol) dropwise under nitrogen atmosphere, The molar ratio of vanillin, bromopropene and anhydrous potassium carbonate is 1:1.2:1.5. After the dropwise addition, gradually heat up to 80°C, condense and reflux, react for 48 hours, filter, remove the solvent under reduced pressure distillation, and add a small amount of water Dilute and extract with ethyl acetate and saturated brine. Combine the organic phases, add anhydrous sodium sulfate, let stand overnight, collect by silica gel dry method, use the mixed solvent of petroleum ether / ethyl acetate (volume ratio 4:1) as the eluent for chromatography column separation, and vacuum at 50°C After drying, 26.8...
Embodiment 3
[0090] 3-methyl-4-hydroxybenzaldehyde monomer allylation reaction, the reaction equation is as follows:
[0091]
[0092] Experimental steps:
[0093]Add 3-methyl-4-hydroxybenzaldehyde (10 mmol) to the flask and dissolve it in 30 milliliters of absolute ethanol, add anhydrous potassium carbonate (15 mmol), stir at room temperature for 30 min, gradually Add bromopropene (12 mmoles) dropwise, the molar ratio of syringaldehyde to bromopropene to anhydrous potassium carbonate is 1:1.2:1.5, after the dropwise addition, gradually warm up to 80°C, condense and reflux, react for 48h, filter, and The solvent was distilled off under reduced pressure, diluted with a small amount of water, and extracted with ethyl acetate and saturated brine. Combine the organic phases, add anhydrous sodium sulfate, let stand overnight, collect by silica gel dry method, use the mixed solvent of petroleum ether / ethyl acetate (volume ratio 10:1) as the eluent for chromatography column separation, and va...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com