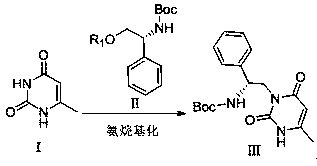
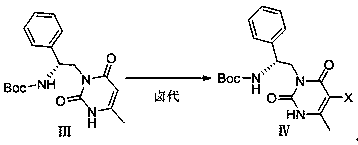
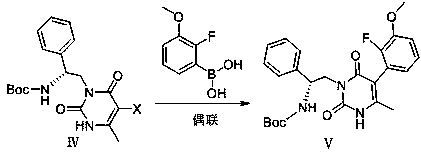
Method for efficiently synthesizing elagolix intermediate
A technology for elagolime and intermediates, which is applied in the field of high-efficiency synthesis of elagolime intermediates, and can solve problems such as expensive starting materials, high process costs, and inapplicability to industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
[0045] Aminoalkylation reaction: at room temperature, put 12.6g of 6-methyluracil, 63g of D-Boc phenylglycinyl mesylate and 32g of 2,6-lutidine into the reaction flask, then add 126mL of N,N - Dimethylformamide, raise the temperature to 55°C, start the heat preservation reaction for 24h, cool down to room temperature, add 100mL isopropyl acetate and 100mL water, stir and separate layers, add 50mL water to the organic layer, wash 3 times, and dry with anhydrous sodium sulfate , filtered, and the filtrate was concentrated to dryness to obtain 30 g of intermediate III, with a weight yield of 86.9% and a HPLC purity of 98%.
[0046] Halogenation reaction: The reaction bottle is protected by nitrogen, add 11.5g of the above intermediate III, 10.8g of iodine chloride and 115mL of methanol at room temperature, heat up to 50°C, keep the temperature for 30h, cool down to room temperature, filter, and rinse the solid with a small amount of methanol. After drying, 15 g of int...
Embodiment 2
[0051] Aminoalkylation reaction: the same operation as in Example 1, except that 32 g of 2,6-lutidine was added instead of 30.4 g of triethylamine, the reaction weight yield was 82%, and the HPLC purity was 96%.
[0052] Halogenation reaction: the same operation as in Example 1, but the halogenation reagent was changed to 10.7g bromine and 10.2g acetic acid, the weight yield was 120%, and the HPLC purity was 95%.
[0053] Coupling reaction: with the operation of embodiment 1, the consumption of palladium acetate is changed from 0.04g to 0.02g, and the consumption of 2-fluoro-3-methoxyphenylboronic acid is changed from 4.3g to 8g, and the reaction weight yield is 95%, HPLC purity 97%.
[0054] Benzyl halide substitution reaction: the same operation as in Example 1, changing the addition of 4.4g potassium carbonate to 3.5g sodium carbonate, changing the addition of 35mL tetrahydrofuran to adding 35mL acetonitrile, the reaction weight yield was 123%, and the HPLC purity was 96%.
...
Embodiment 3
[0057] Aminoalkylation reaction: the operation was the same as in Example 1, except that 32 g of 2,6-lutidine was added instead of 30 g of pyridine, the reaction weight yield was 84%, and the HPLC purity was 94%.
[0058] Halogenation reaction: the same operation as in Example 1, changing the addition of 10.8g iodine chloride to 20.2g iodine chloride, the reaction weight yield was 113%, and the HPLC purity was 94%.
[0059] Coupling reaction: with the operation of embodiment 1, the consumption of palladium acetate is changed from 0.04g to 0.01g, and the consumption of 2-fluoro-3-methoxyphenylboronic acid is changed from 4.3g to 3.2g, and the reaction weight is obtained Yield 92%, HPLC purity 95%.
[0060] Benzyl halide substitution reaction: the same operation as in Example 1, the addition of 4.4g of potassium carbonate was changed to 2.5g of cesium carbonate, and the addition of 35mL of tetrahydrofuran was changed to 35mL of 1,4-dioxane. The reaction weight yield was 120%. H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com