High efficiency organic small molecule receptor material, preparation method and application thereof
A small molecule acceptor and organic technology, applied in the field of A-D-A type non-fullerene organic small molecule acceptor material and its preparation, achieving the effect of low production cost and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
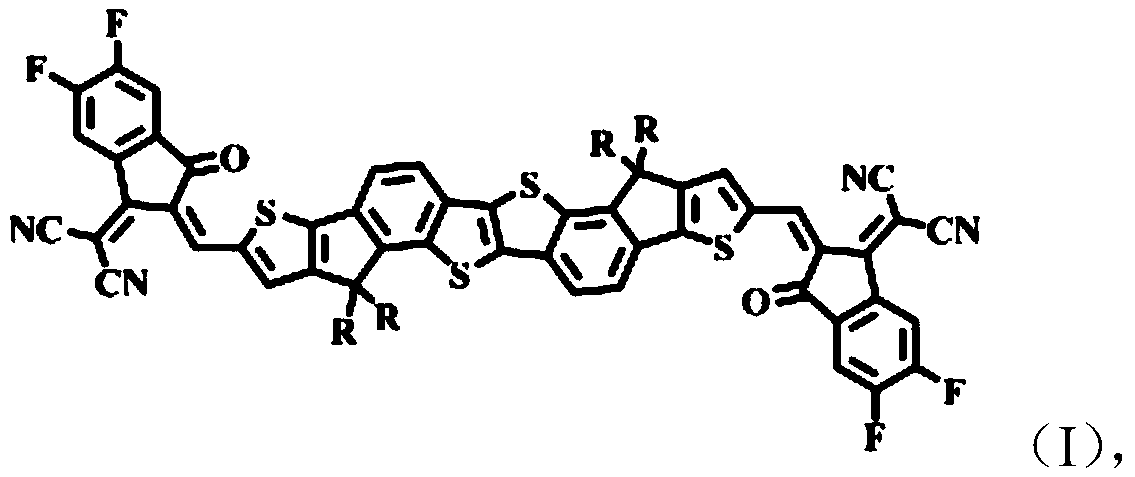
[0047] A kind of chemical structural formula is the non-fullerene organic small molecule acceptor material of Z1, and its synthetic route is as follows:
[0048]
[0049] (1) chemical structural formula is the synthesis of the intermediate of 1:
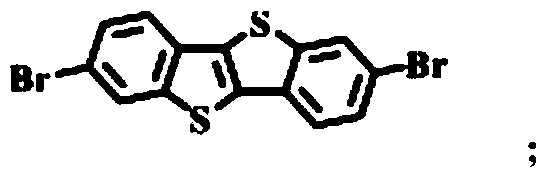
[0050] Add Br-BTBT-Br (1g, 2.51mmol) in 100ml two-necked round-bottomed flask, biboronic acid pinacol ester (2.54mg, 10mmol), bistriphenylphosphine palladium dichloride (100mg), excess potassium acetate ( 2.5 g) and 50 mL of toluene; the reaction bottle was sealed under a nitrogen atmosphere for multiple deoxygenation operations and the reaction mixture was stirred at 85°C in the dark for 24 hours; the toluene was spin-dried, dissolved with a small amount of dichloromethane, washed twice with water, The aqueous phase was back-extracted once with dichloromethane, and this operation was repeated twice. Dry with anhydrous magnesium sulfate, filter and spin dry; and use mixed solvent (petroleum ether / ethyl acetate, v / v=5:1) as eluent...
Embodiment 2
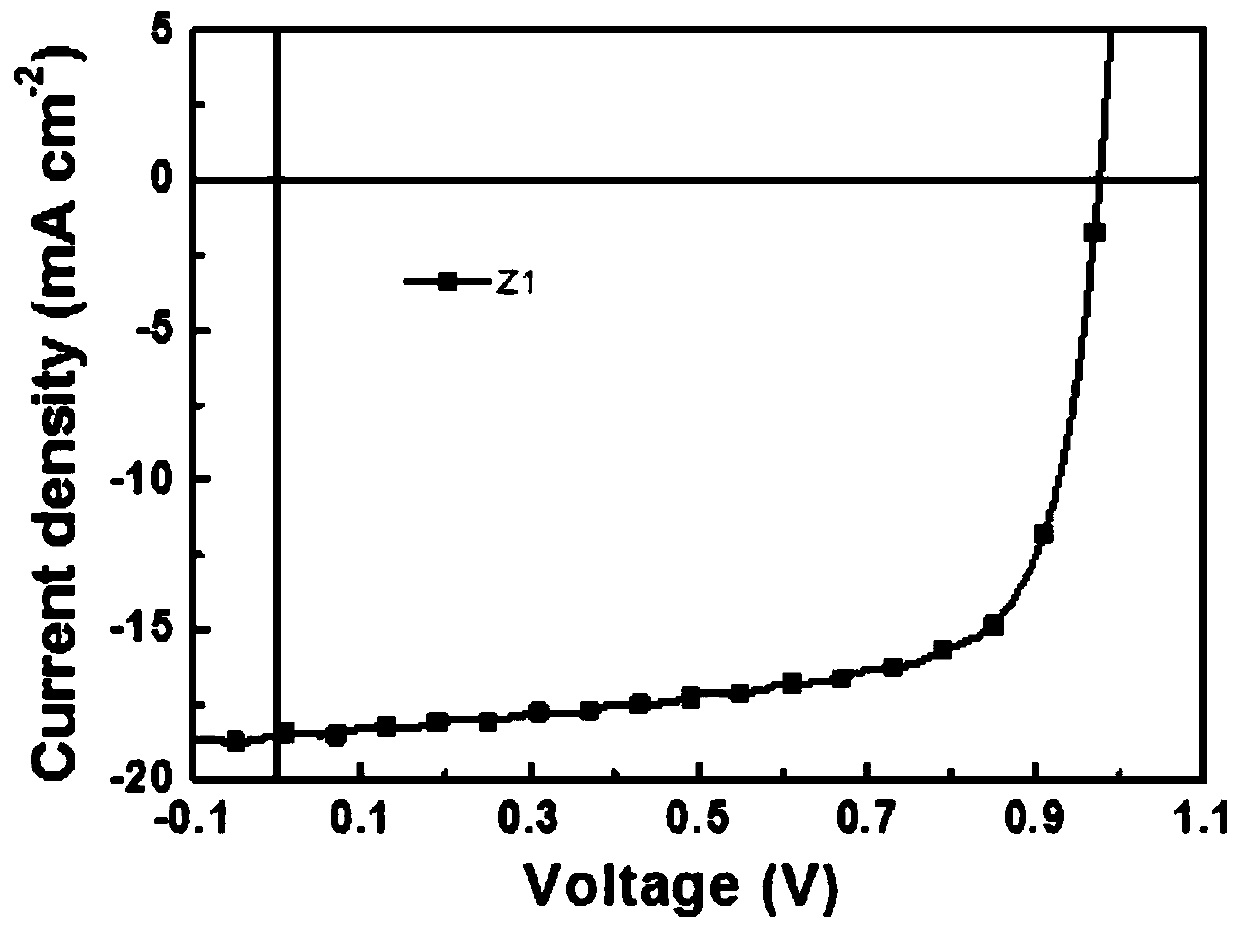
[0060] Preparation of binary solar cells based on non-fullerene organic small molecule Z1 acceptor materials and their photovoltaic performance tests:
[0061] First, clean the purchased ITO conductive glass: ultrasonic cleaning with cleaning solution, ultrapure water, acetone, ethanol, and isopropanol for 15 to 30 minutes, then dry it in a drying oven at 100°C for 10 minutes, take it out, and cool it Then UV and ozone treatment for 15 minutes for later use. Then configure the ZnO precursor solution: weigh a certain amount of zinc acetate dihydrate and ethanolamine, dissolve in 2-methoxyethanol, and obtain a concentration of zinc acetate dihydrate of 0.75mol L -1 , the concentration of ethanolamine is 0.75mol L -1 The solution was then placed in a glove box and stirred at room temperature for more than 8 hours, and set aside. Put the previously treated ITO conductive glass into a PPM glove box with a water oxygen index less than 1, spin-coat the ZnO precursor solution, take ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com