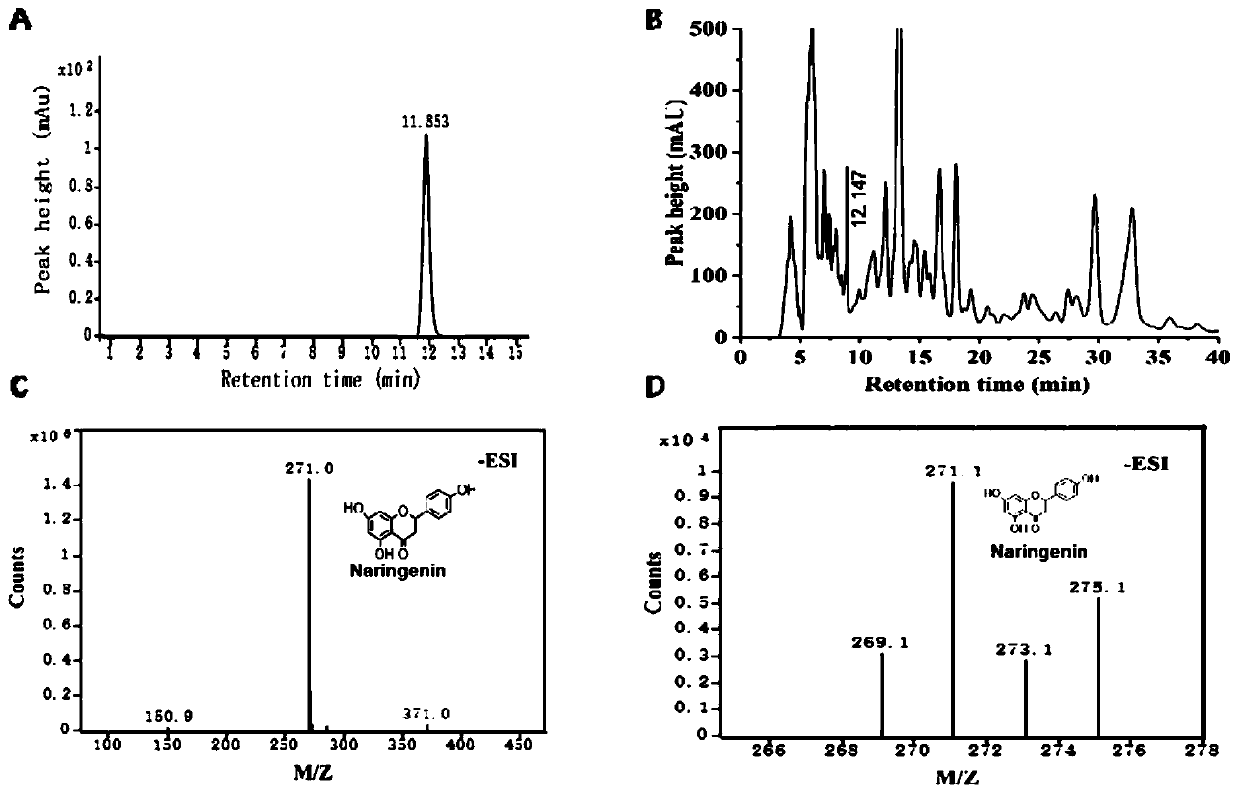
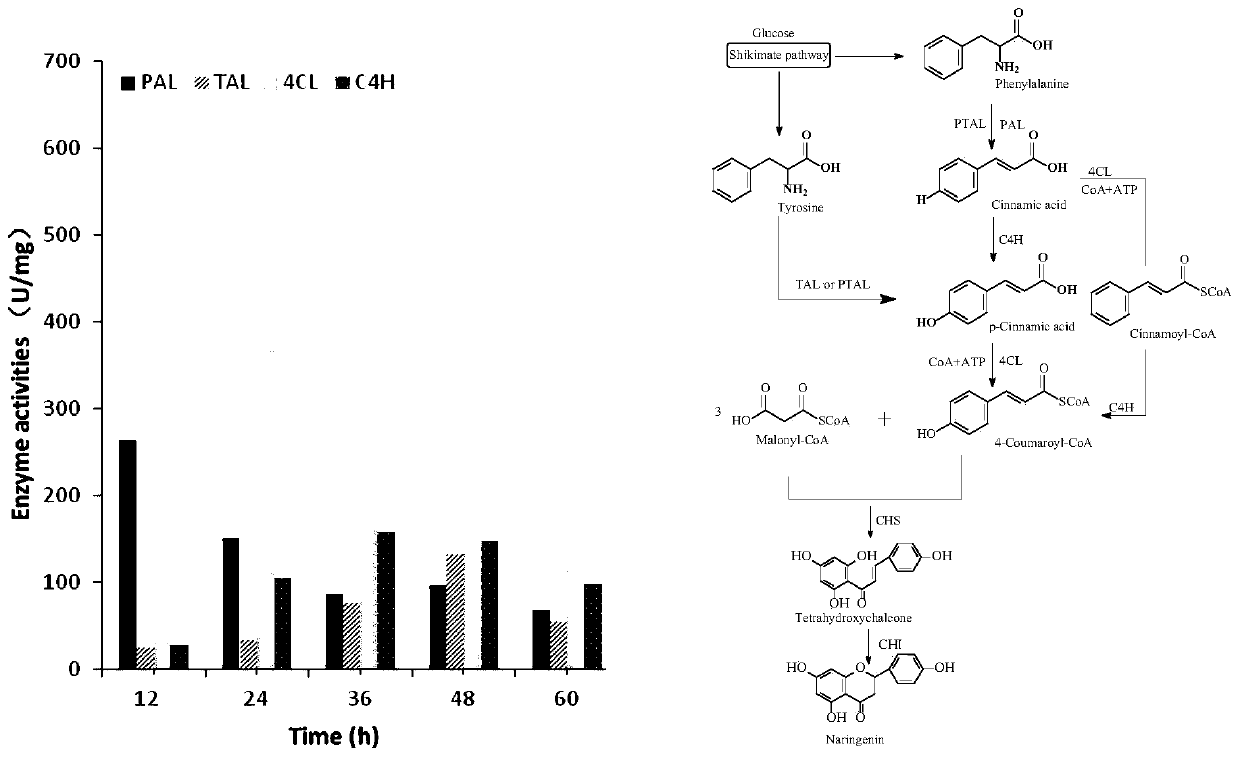
Naringenin production method based on phomopsis cell fermentation
A technology of P. phomophie and cell fermentation, which is applied in the fields of molecular biology and biology, can solve the problems of inability to guarantee product chirality, functional verification of enzyme activity, long plant growth cycle, etc., achieves good application prospects, overcomes long cycle, Easy to use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Activation of wild-type Phomopsis species
[0037] The wild-type Phomopsis species is isolated from the stem endothelium of Euphorbiaceae Chongyang wood. The activation of the strain is to scrape a little Phomopsis mycelium from the PDA solid medium stored in the test tube and inoculate it into a 50mL PDA liquid culture medium. culture medium at 28°C and 180rpm for 48h, then transfer 2mL of mycelia liquid from the above bacterial liquid to 50mL of fresh PDA liquid medium, and continue to cultivate at 28°C and 180rpm for 36h to obtain protoplasts Prepared bacteria solution. Described PDA liquid culture medium is made of potato 200g L -1 and glucose 20g L -1 composition.
[0038] The PDA liquid culture medium in the above-mentioned example can also be used to add 10g L -1 Glucose was replaced by a synthetic medium consisting of glucose 10 g L -1 , ammonium sulfate 2g L -1 , sodium dihydrogen phosphate 3g L -1 , yeast extract 1g L -1 , magnesium sulfate ...
Embodiment 2
[0039] Embodiment 2: the preparation of protoplast
[0040] (1) Use a nylon membrane to filter the Phomopsis mycelia liquid cultured for 36 hours to obtain mycelia, and then use 0.6M MgSO 4 The mycelium was cleaned, and 1 g of wet mycelium was taken in 10 mL of lysate (20 mg Trichoderma, 20 mg Yatalase, 0.8 M NaCl to 10 mL), and incubated at 28° C. and 80 rpm for 13 h.
[0041] (2) Take it out after incubation for 13h, centrifuge at 3000rpm for 10min, discard the supernatant, and wash with 5mL STC buffer (sorbitol 1.2mol L -1 , CaCl 2 10mM·L -1 , Tris·HCl 10mM·L -1 , pH 7.5) to obtain the protoplast suspension after resuspension, centrifuge the suspension at 5000rpm for 10min. At this time, the protoplast floats on the top of the liquid surface of the centrifuge tube, and the impurities settle to the bottom of the tube. Protoplasts in a new 1.5mL centrifuge tube, diluted to 10 8 pcs mL -1 spare.
Embodiment 3
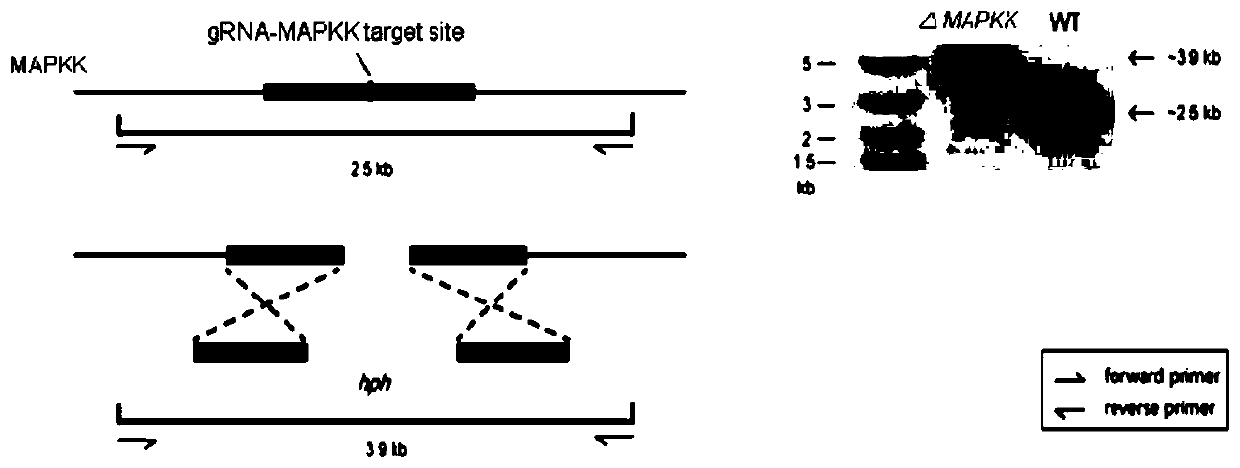
[0042] Embodiment 3: Construction of MAPKK gene knockout plasmid
[0043] Using the pCT74 plasmid as the backbone, the CDS region of the Cas9 protein was amplified from the pX330 plasmid by PCR, and the amplified DNA fragment was seamlessly cloned into the front of the fluorescent protein in the pCT74 plasmid, so that the Cas9 protein and the fluorescent protein were seamlessly fused into one protein , the first plasmid pCT74-Cas9 was constructed; the U6 promoter of Aspergillus fumigatus was amplified by PCR using the Aspergillus fumigatus genome as a template, and the U6 promoter and the sgRNA backbone of MAPKK were fused into a DNA fragment and cloned seamlessly into the first The XhoI restriction site of plasmid pCT74-Cas9 was used to construct the second plasmid pCT74-Cas9-sgRNA; using the website http: / / zifit.partners.org / ZiFiT / CSquare9Nuclease.aspx Design the gRNA sequence of the target gene (sequence: GGGGCGTTCATCATGGGGAC, SEQ ID No.1), select two complementary gRNA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com