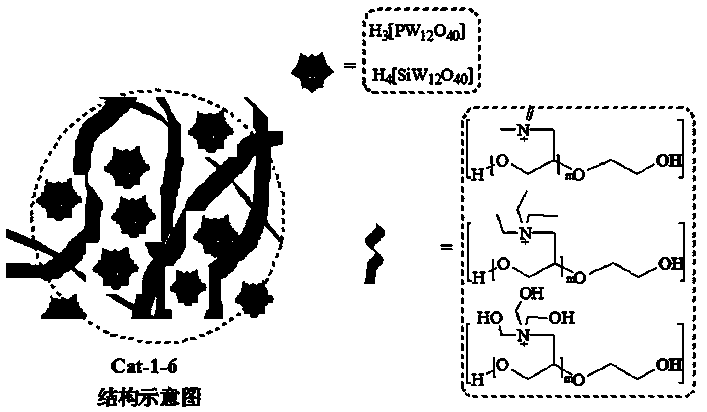
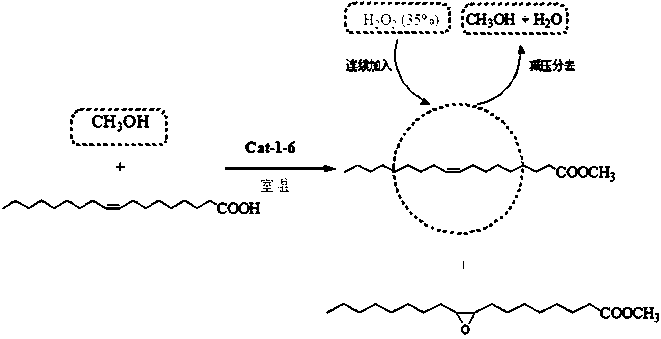
Hydroxyl polyether functionalized heteropolyacid polyionic liquid and application thereof in synthesis of epoxy methyl oleate by one-pot process
A technology of hydroxyl polyether functional and epoxy methyl oleate, which is applied in the direction of catalytic reaction, chemical instruments and methods, organic compound/hydride/coordination complex catalyst, etc., which can solve the problems of unstable product quality, equipment corrosion, Poor thermal stability and other problems, to achieve good surface amphiphilic activity, easy to recycle, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
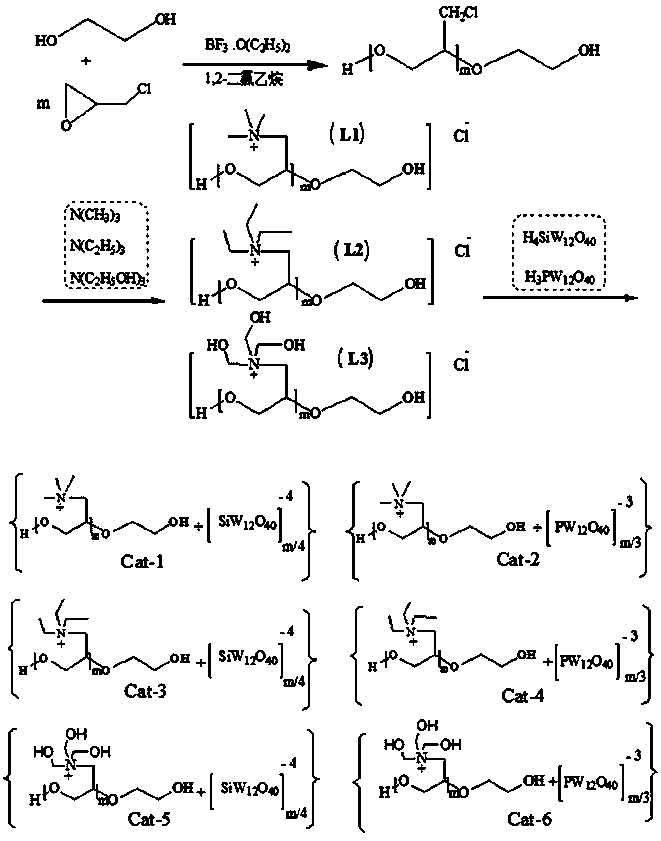
[0033] Embodiment 1: the synthesis of hydroxyl polyether
[0034] In a reaction flask equipped with magnetic stirring, add 10mL 1,2-dichloroethane, 3.5mL ethylene glycol, 1.5mL boron trifluoride ether, cool to 0-5°C and stir for 0.5 h, dropwise add 25ml epoxy Chloropropane, add 15ml 1,2-dichloroethane three times in the middle and keep it warm for 6 hours. Water was added to terminate the reaction, the organic phase was separated, washed with water until neutral, dried, and rotary evaporated to obtain 25.7 g of a colorless viscous liquid target product with a yield of 90.7%.
[0035] FT-IR (KBr), ν / cm -1 : 3460, 2918, 1430, 1121, 748.
Embodiment 2
[0036] Embodiment 2: the synthesis of quaternary ammonium salt L1-3
[0037] A. Under the protection of nitrogen, add 30 mL of acetone and 6.0 g of hydroxypolyether to the reaction bottle, raise the temperature to 45 °C, add 0.2 g of trimethylamine dropwise, and keep the reaction for 12 h after the addition, and vacuum dry to constant weight after rotary evaporation to obtain Hydroxy polyether quaternary ammonium salt L1, yield 93%.
[0038] FT-IR (KBr), ν / cm -1 : 3376, 2923, 1438, 1125, 744.
[0039] B. Replace 0.2g of trimethylamine in A with 0.3g of triethylamine, and the others are the same as in A to obtain hydroxy polyether quaternary ammonium salt L2 with a yield of 95%.
[0040] FT-IR (KBr), ν / cm -1 : 3457, 2924, 1438, 1124, 752.
[0041] C. Replace 0.2g of trimethylamine in A with 0.5g of triethanolamine, and the others are the same as in A to obtain hydroxy polyether quaternary ammonium salt L3 with a yield of 96%.
[0042] FT-IR (KBr), ν / cm -1 : 3434, 2930, 14...
Embodiment 3
[0043] Embodiment 3 [C 8 h 20 o 3 N + ] 3n [PW 12 o 40 -3 ] n (Cat-1), [C 8 h 20 o 3 N + ] 4n [SiW 12 o 40 -4 ] n (Cat-2) synthesis
[0044] After fully dissolving 0.7g of hydroxy polyether quaternary ammonium salt L1 in deionized water, slowly add an aqueous solution containing 2.0g of silicotungstic acid or 1.92g of phosphotungstic acid dropwise in 3 times, react at room temperature for 24 hours, and centrifuge The crude product of Cat-1 or Cat-2 was obtained, and the crude product was vacuum-dried at 80-85°C to constant weight. The quality of the obtained product was 1.9 g or 2.1 g, and the yields were 82% and 84%, respectively.
[0045] FT-IR (KBr) (Cat-1), ν / cm -1 : 3358, 2965, 1018, 970, 789, 535.
[0046]FT-IR (KBr) (Cat-2), ν / cm -1 : 3348, 2980, 1085, 971, 877.
[0047] There is no characteristic peak of phosphotungstic acid or silicotungstic acid in polyionic liquid XRD, indicating that polyionic liquid exists in an amorphous form.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com