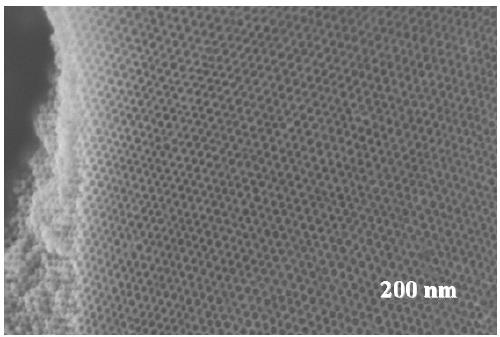
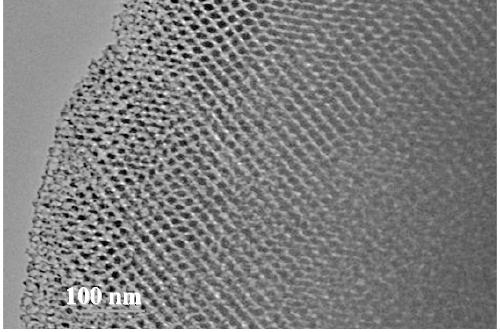
Method for synthesizing large-aperture mesoporous bimetallic oxide semiconductor gas-sensitive material
A technology of double metal oxide and synthesis method, applied in zirconia, tungsten oxide/tungsten hydroxide, nanotechnology, etc., can solve the problems of poor stability, difficult industrial production, difficult control of synthesis process, etc., and achieve good sensitivity and Effects of selectivity, fast response and recovery time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Add 0.10 g amphiphilic block copolymer polyethylene oxide- b- Polystyrene (PEO 112 - b -PS 236 ,M n =27948 gmol -1 ) was dissolved in 5 g tetrahydrofuran, and stirred at room temperature for 0.5 h to obtain solution A; 0.10 g tungsten chloride and 0.20 g n-propoxide zirconium were dissolved in 2.0 g ethanol, and stirred at room temperature for 0.5 h to obtain solution B; solution A was mixed with B is mixed evenly, and after stirring for 2 hours at room temperature, a homogeneous mixed solution is finally obtained;
[0025] (2) The homogeneous solution was transferred to a watch glass and volatilized at room temperature for 24 h; then the watch glass was transferred to a 40°C oven to further evaporate the solvent for 24 h, and then transferred to a 100°C oven for curing for 24 h. Finally, the composite film is scraped off from the watch glass and ground to obtain a solid powder;
[0026] (3) The obtained solid powder was placed in a tube furnace, and calcined ...
Embodiment 2
[0028] (1) Add 0.10 g amphiphilic block copolymer polyethylene oxide- b -Polymethylmethacrylate (PEO 123 - b -PMMA 184 ,M n =35729 gmol -1 ) was dissolved in 5 g tetrahydrofuran, and stirred at room temperature for 0.5 h to obtain solution A; 0.15 g cobalt chloride and 0.3 g n-propoxide zirconium were dissolved in 2.0 g ethanol, and stirred at room temperature for 0.5 h to obtain solution B; solution A was mixed with B is mixed evenly, and after stirring for 2 hours at room temperature, a homogeneous mixed solution is finally obtained;
[0029] (2) The homogeneous solution was coated on a quartz plate by spin coating, and volatilized at room temperature for 24 h; then transferred to a 40°C oven to further volatilize the solvent for 24 h, and then transferred to a 100°C oven for curing 24 h. Finally, the composite film is scraped off from the quartz plate and ground to obtain a solid powder;
[0030] (3) The obtained solid powder was placed in a tube furnace, and calcine...
Embodiment 3
[0032] (1) Add 0.10 g amphiphilic block copolymer poly-(4-vinylpyridine)- b - Polystyrene (P4VP 84 - b -PS 113 , Mn=19356 g mol -1 ) was dissolved in 5 g of dichloromethane solution, stirred at room temperature for 0.5 h to obtain solution A; 0.125 g of tin tetrachloride and 0.25 g of titanium isopropoxide were dissolved in 2.0 g of ethanol, and stirred at room temperature for 0.5 h to obtain solution B; Mix solutions A and B evenly, and stir at room temperature for 2 hours to finally obtain a homogeneous mixed solution;
[0033] (2) Apply the homogeneous solution on the quartz plate by pulling method, and volatilize at room temperature for 24 h; then transfer it to a 40°C oven to further volatilize the solvent for 24 h, and then transfer to a 100°C oven for curing 24 h. Finally, the composite film is scraped off from the quartz plate and ground to obtain a solid powder;
[0034] (3) The obtained solid powder sample was placed in a tube furnace, and calcined in a nitroge...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com