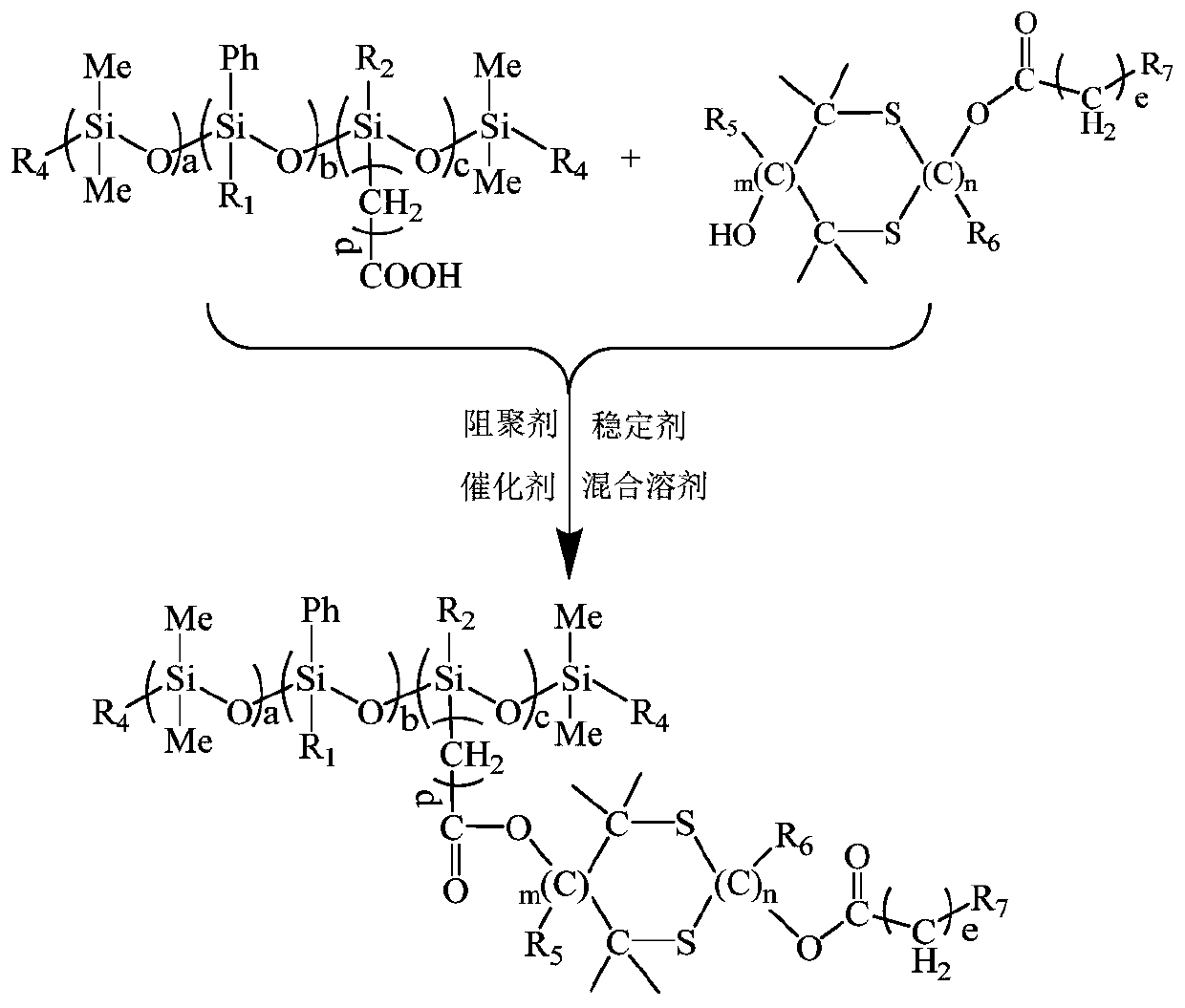
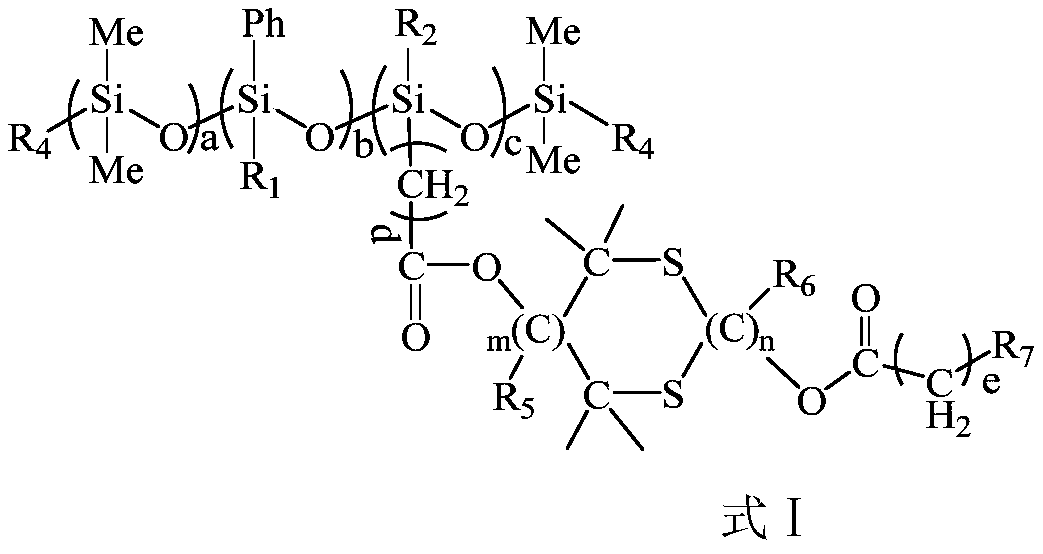
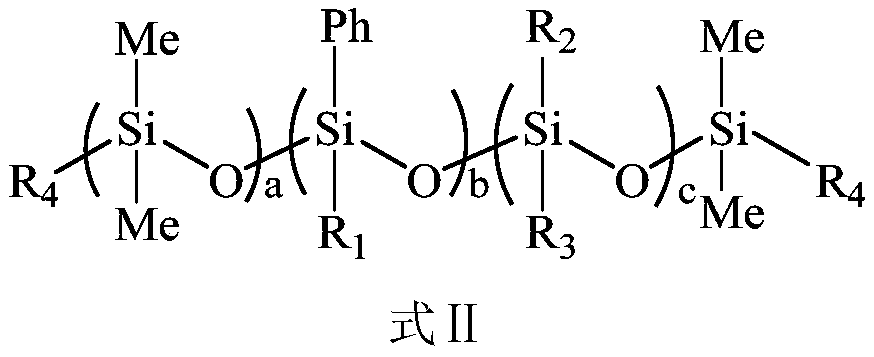
Ultraviolet-curable polysiloxane and preparation method and application thereof
A polysiloxane and ultraviolet light technology, used in electrical components, circuits, semiconductor devices, etc., can solve the problems of difficult industrial production, many unstable factors, harsh reaction conditions, etc., and achieve excellent storage stability and mechanical properties. , non-toxic, odorless, biocompatibility, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] A UV-curable polysiloxane C 223 h 289 Si 18 o 37 S 10 (Theoretical value: C 61.08%, H6.60%, O13.50%, S 7.30%) Its structural formula is as follows:
[0076]
[0077] Its preparation method is:
[0078] When equipped with stirring paddle, condenser, N 2 Add 68.70g, 0.02mol carboxyphenyl silicone oil, 206.20g toluene, 51.55g ethanol, 0.10g stannous dioctoate, 0.52g methylthiouracil, 1.03 p-hydroxyanisole to the gas inlet and the four-necked bottle of the thermometer. g and photocuring modified sulfur-containing heterocyclic monomer 20.60g, 0.1mol, use a water separator to remove water, react at 90°C for 2 hours, add 4A molecular sieve (5.15g), continue the heat preservation reaction for 7 hours, and pass the acid value test , when the acid value is less than 0.1mg KOH / g, filter with suction, wash with ice water twice, take the oil layer, and rectify for 0.5h at a vacuum degree of -0.095~-0.10MPa and a temperature of 90°C to obtain a colorless, transparent viscous...
Embodiment 2
[0090] A UV-curable polysiloxane C 567 h 946 Si 34 o 93 S 30 (Theoretical value: C 61.02%, H8.48%, O13.34%, S 8.61%) Its structural formula is as follows:
[0091]
[0092] Its preparation method is:
[0093] When equipped with stirring paddle, condenser, N 2 In the four-necked bottle of gas inlet and thermometer, add carboxyphenyl silicone oil 25.33g, 0.0053mol, ethyl acetate (209.43g), ethanol (27.92g), bismuth laurate (0.10g), 2-methylthio-4 -Pyrimidinone (0.21g), polymerization inhibitor 701 (0.35g) and UV-curable modified sulfur-containing heterocyclic monomer, 44.48g, 0.1mol, use a water separator to remove water, and react at 80°C for 5h Finally, add anhydrous magnesium sulfate (1.40g), continue the insulation reaction for 5 hours, pass the acid value test, when the acid value <0.1mgKOH / g, filter with suction, wash with ice water 2 times, take the oil layer, and reach - After rectification at 0.095~-0.10MPa and temperature 80℃ for 1h, a light yellow transparen...
Embodiment 3
[0105] A UV-curable polysiloxane C 354 h 592 Si 21 o 60 S 20 (theoretical value: C 60.44%, H8.42%, O13.66%, S 9.11%), its structural formula is as follows:
[0106]
[0107] Its preparation method is:
[0108] When equipped with stirring paddle, condenser, N 2 Add 24.17g of carboxyphenyl silicone oil, 0.006mol, n-hexane (56.02g), tetrahydrofuran (16.81g), tin chloride (0.04g), 6-aza-2-sulfur Uracil (0.45g), polymerization inhibitor 705 (0.84g), and UV-curable modified sulfur-containing heterocyclic monomer 31.85g, 0.1mol, use a water separator to remove water, and react at 85°C for 3h , add anhydrous calcium oxide (4.50g), continue the heat preservation reaction for 6 hours, pass the acid value test, when the acid value is <0.1mgKOH / g, filter with suction, wash with ice water for 2 times, take the oil layer, and reach -0.095 ~-0.10MPa and temperature 85°C after rectification for 0.8h, a light yellow transparent sticky substance was obtained.
[0109] Wherein, the st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Acid value | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com