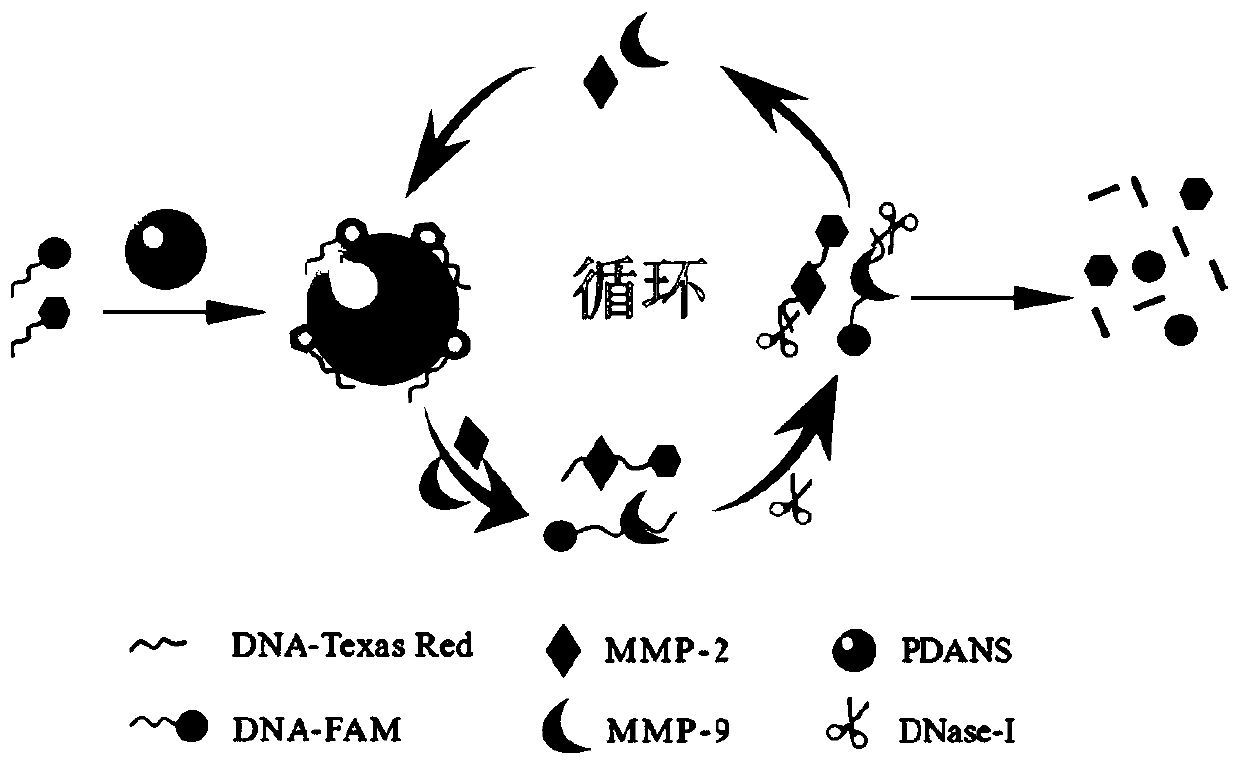
System for simultaneously detecting matrix metalloproteinase-9 and matrix metalloproteinase-2, preparation method thereof and application thereof
A matrix metal and protease technology, applied in the field of biomedical detection, can solve the problems of limited MMP-9 and MMP-2 detection, high detection cost, and complicated steps, and achieve the effects of saving sample volume, improving sensitivity, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
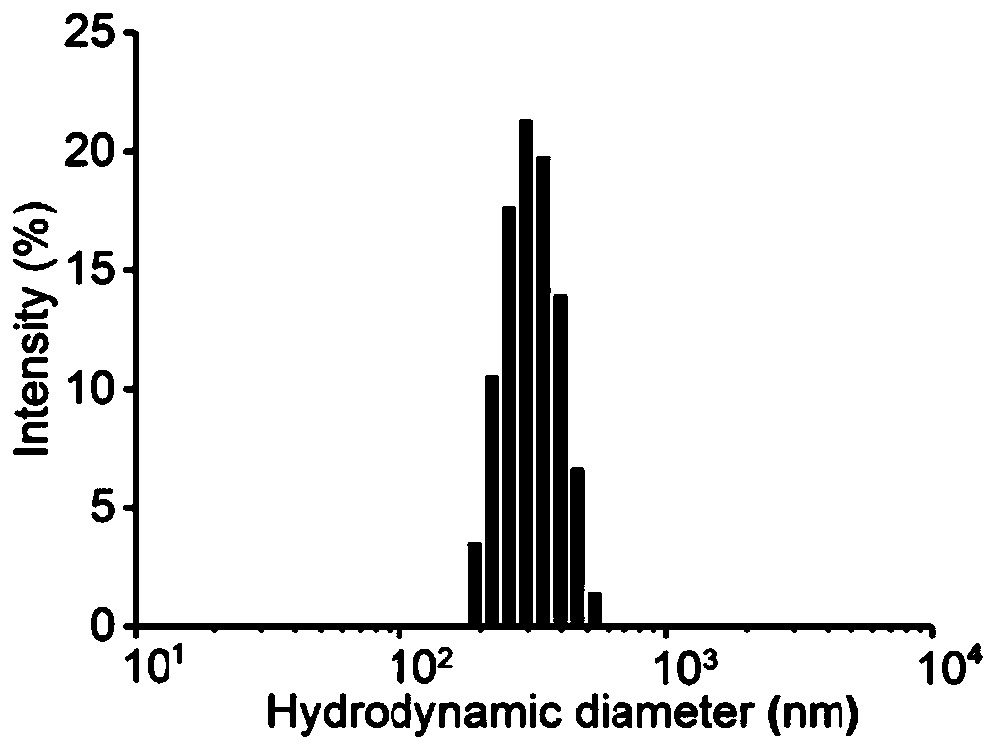
[0047] Successful verification of PDANS synthesis
[0048] Prepare PDANS:
[0049] (1) Prepare 100mL 10mM Tris-HCl (pH 7.4) with ultrapure water, add Tris-HCl and isopropanol (40 mL) into the beaker, dilute with ammonia water, add a small amount to the beaker several times, and test with pH test paper. The pH value of the final solution was about 8.0, and the magnetic stirrer was stirred slowly for 30 minutes to fully mix the solution.
[0050] (2) Subsequently, 100 mg of dopamine hydrochloride was weighed and dissolved in 2 mL of ultrapure water, and the dissolved dopamine hydrochloride was slowly added into the solution, stirred for 30 h, and then stopped.
[0051] (3) Transfer the solution obtained in step (2) to a centrifuge tube, centrifuge at 10000rpm for 10min, discard the supernatant, dissolve the precipitate in ultrapure water, vortex mix, centrifuge, discard the supernatant, repeat several times, until the supernatant is clear.
[0052] (4) After centrifugation an...
Embodiment 2
[0058] Building PDANS-aptamers nanosensors
[0059] 1. Modified FAM fluorescein (MMP-9-aptamer-FAM) at the 5' end of MMP-9-aptamer, and modified Texas Red fluorescein (MMP-2-aptamer-Texas Red) at the 5' end of MMP-2-aptamer, The sequence was synthesized by Shanghai Sangong Technology Co., Ltd.
[0060] name Nucleotide sequence MMP-9-aptamer-FAM 5'-FAM-tacggccgcacgaaaaggtgccccataactcaatgccga-3' MMP-2-aptamer-Texas Red 5'-Texas Red-tcgccgtgtaggattaggccaggtatgggaacccggtaac-3'
[0061] Construction of the PDANS-aptamers nanosensor involves the following steps:
[0062] (1) Preparation of samples: Prepare MMP-9-aptamer-FAM and MMP-2-aptamer-TexasRed standard solutions of 1000 nM with ultrapure water for use; prepare PDANS standard solution with ultrapure water at a concentration of 2 mg / mL.
[0063] (2) Reaction: Add MMP-9-aptamer-FAM and MMP-2-aptamer-Texas Red standard solutions in sequence to make the final concentrations 100nM respectively; then ...
Embodiment 3
[0069] Investigate the detection sensitivity experiment of the whole detection system to MMP-9 and MMP-2
[0070] 1. The sequence required for this experiment (as in Example 2):
[0071] 2. Investigate the sensitivity of MMP-9 and MMP-2 standard solutions based on DNase-I amplification system,
[0072] Include the following steps:
[0073] (1) Preparation of samples: prepare MMP-9 concentration of 0.48~120ng / mL, so that the concentrations are 0.48 ng / mL, 0.96ng / mL, 2.4ng / mL, 4.8ng / mL, 12ng / mL, 24ng / mL, 60ng / mL, 120ng / mL; MMP-2 concentration 1.28~320ng / mL, so that the concentrations are 1.28ng / mL, 2.56ng / mL, 6.4ng / mL, 12.8ng / mL, 32ng / mL, 64ng / mL , 160ng / mL, 320ng / mL; prepare DNase-I enzyme 2U / μL in ultrapure water.
[0074] (2) Perform the reaction: add MMP-9-aptamer-FAM and MMP-2-aptamer-Texas Red standard solutions in sequence to make the final concentration 100nM respectively, then add PDANS to make the final concentration 0.3 mg / mL, buffer ion Concentration of 5mM CaCl 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com