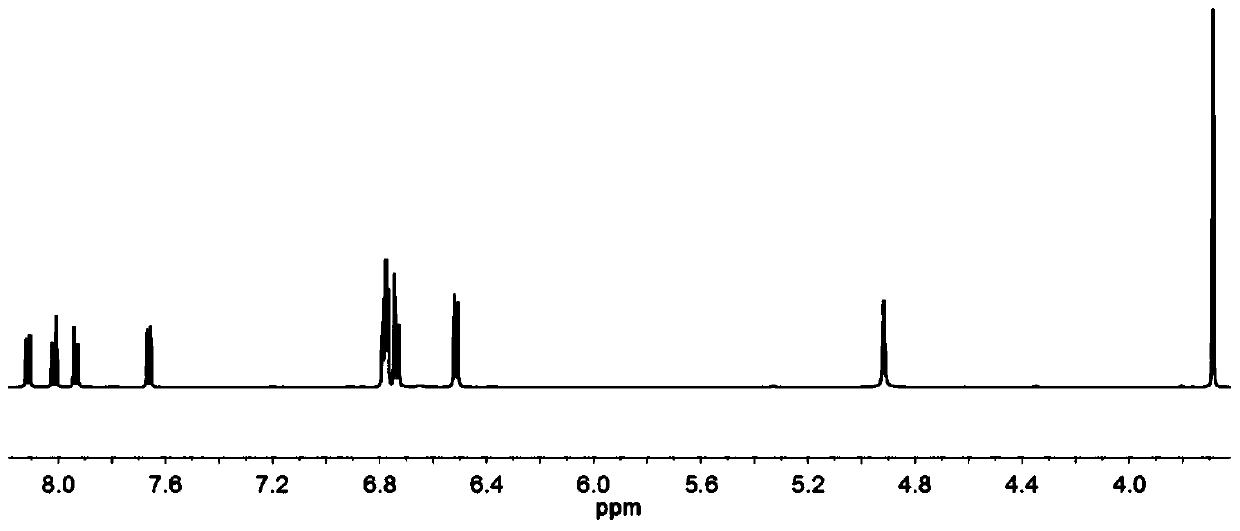
Diamine compound containing diarylamine-pyrene structure, and preparation method thereof, polyamide, polyimide and applications thereof
A technology of amine compounds and polyimides, applied in the field of electronically controlled fluorescence, which can solve the problems of low solid-state fluorescence intensity and reduced stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The present invention also provides the preparation method of the diamine compound containing bisarylamine-pyrene structure described in the above technical scheme, comprising the following steps:
[0040] (1) After mixing p-fluoronitrobenzene, 4-methoxyaniline, triethylamine and solvent, in a protective atmosphere, carry out nucleophilic substitution reaction to obtain 4-nitro-4'-methoxydiphenylamine ;
[0041] (2) After mixing the 4-nitro-4'-methoxydiphenylamine, 1,6-dibromopyrene, potassium carbonate, copper powder, 18-crown-6 and solvent, in a protective atmosphere, Carry out the Ullmann reaction to obtain two (4-nitrophenyl)-bis(4'-methoxyphenyl)-1,6-diaminopyrene;
[0042] (3) Mix the bis(4-nitrophenyl)-bis(4'-methoxyphenyl)-1,6-diaminopyrene, Pd / C catalyst and solvent, and heat to 70-90°C , and then adding hydrazine hydrate for reduction reaction to obtain a diamine compound containing a bisarylamine-pyrene structure.
[0043] In the invention, after mixing p-...
Embodiment 1
[0121] Mix 40g (324.8mmoL) of p-methoxyaniline, 35.2540g (249.9mmoL) of p-fluoronitrobenzene, 32.8672g (324.8mmoL) of triethylamine and 273mL of dimethyl sulfoxide, stir and nitrogen Under protected conditions, react at 85°C for 36 hours; after the reaction is completed, cool to room temperature, discharge the material in ice water, stir well, and filter to obtain the crude product; mix the crude product with N,N-dimethylacetamide and ethanol (Volume ratio 1:2) was prepared into a hot saturated solution, the temperature of the hot saturated solution was 90°C, then naturally cooled to room temperature, filtered, and the resulting solid was vacuum-dried at 80°C to obtain 50.1 g of orange 4-nitro- 4'-methoxy-diphenylamine, the yield is 82.1%;
[0122] 40g (163.8mmoL) of 4-nitro-4'-methoxy-diphenylamine, 23.59g (65.52mmoL) of 1,6-dibromopyrene, 33.31g (524.2mmoL) of copper powder, 72.45g (524.2mmoL) ) of potassium carbonate, 22.51g (85.18mmoL) of 18-crown-6 and 196mL of o-dichlor...
Embodiment 2
[0128] With 0.6268g (1mmoL) two (4-aminophenyl)-two (4'-methoxyphenyl)-1,6-diaminopyrene (prepared by Example 1), 0.1722g (1mmoL) 1, 4-Cyclohexanedioic acid, 0.2397g CaCl 2 , 1.6mL triphenyl phosphite, 3.8mL pyridine and 3.1mL N-methylpyrrolidone were mixed, and reacted at 110°C for 3.5h under a nitrogen atmosphere; after the reaction was completed, cooled to room temperature, and discharged into 100mL ethanol , and then filtered to obtain a yellow fibrous substance; the obtained yellow fibrous substance was washed with ethanol, water, and ethanol in turn for 30 minutes, and then dried in a vacuum oven at 90°C to obtain 1,4-cyclohexanedioic acid poly Amide, the mass is 0.7802g.
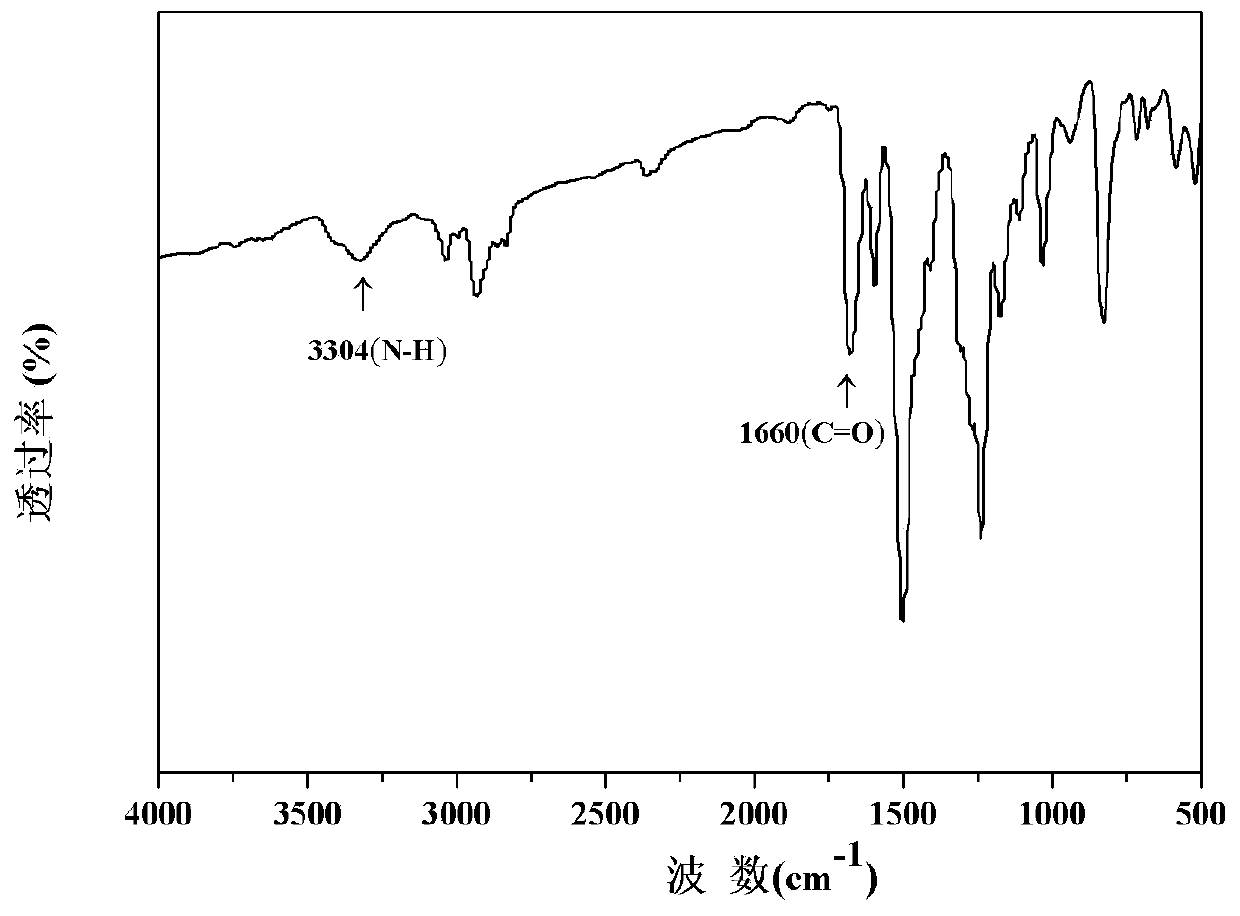
[0129] Test the infrared spectrum of the product obtained in this embodiment, the results are as follows figure 2 shown. Among them, 3304cm -1 N-H stretching vibration absorption peak, 1660cm -1 It is the C=O stretching vibration absorption peak, indicating that the polyamide obtained in this ex...
PUM
| Property | Measurement | Unit |
|---|---|---|
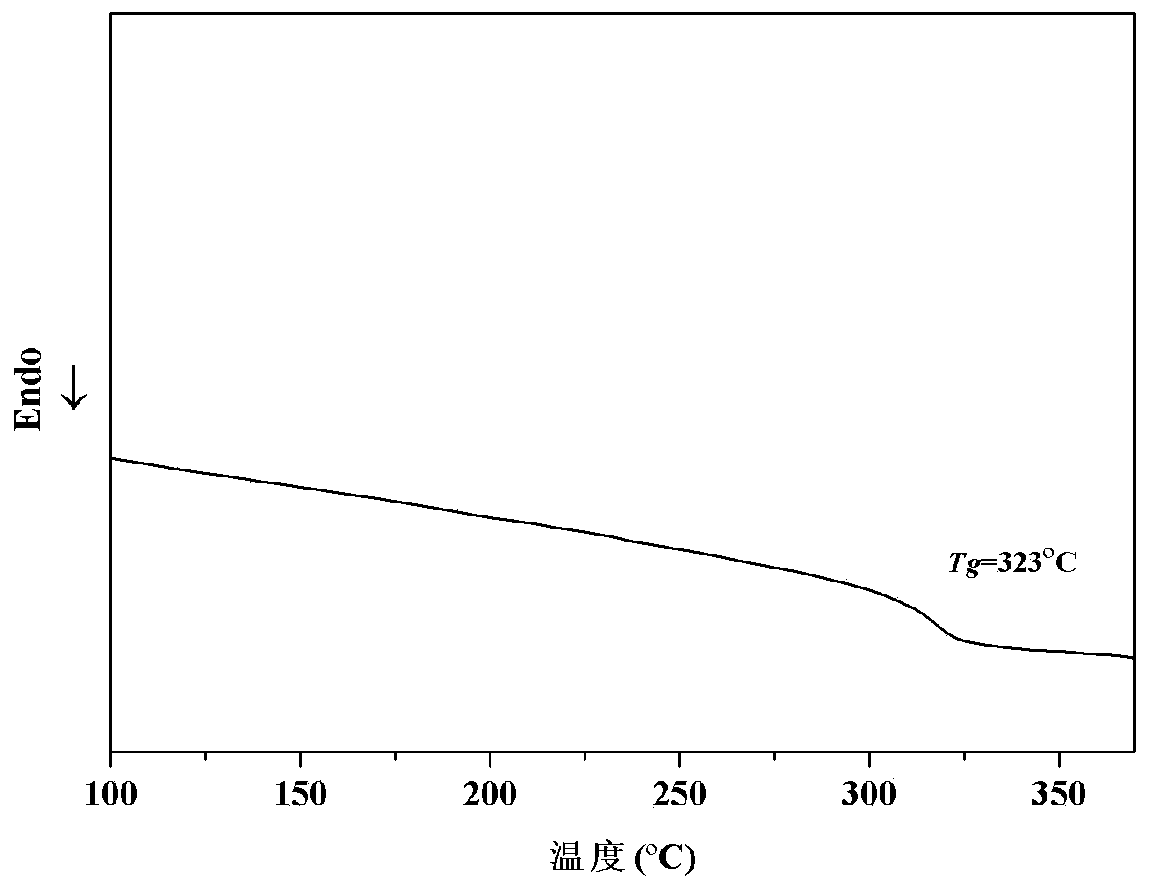
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com