Fluorene derivative and electronic device
A technology of fluorene derivatives and electronic devices, applied in the field of organic optoelectronic materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
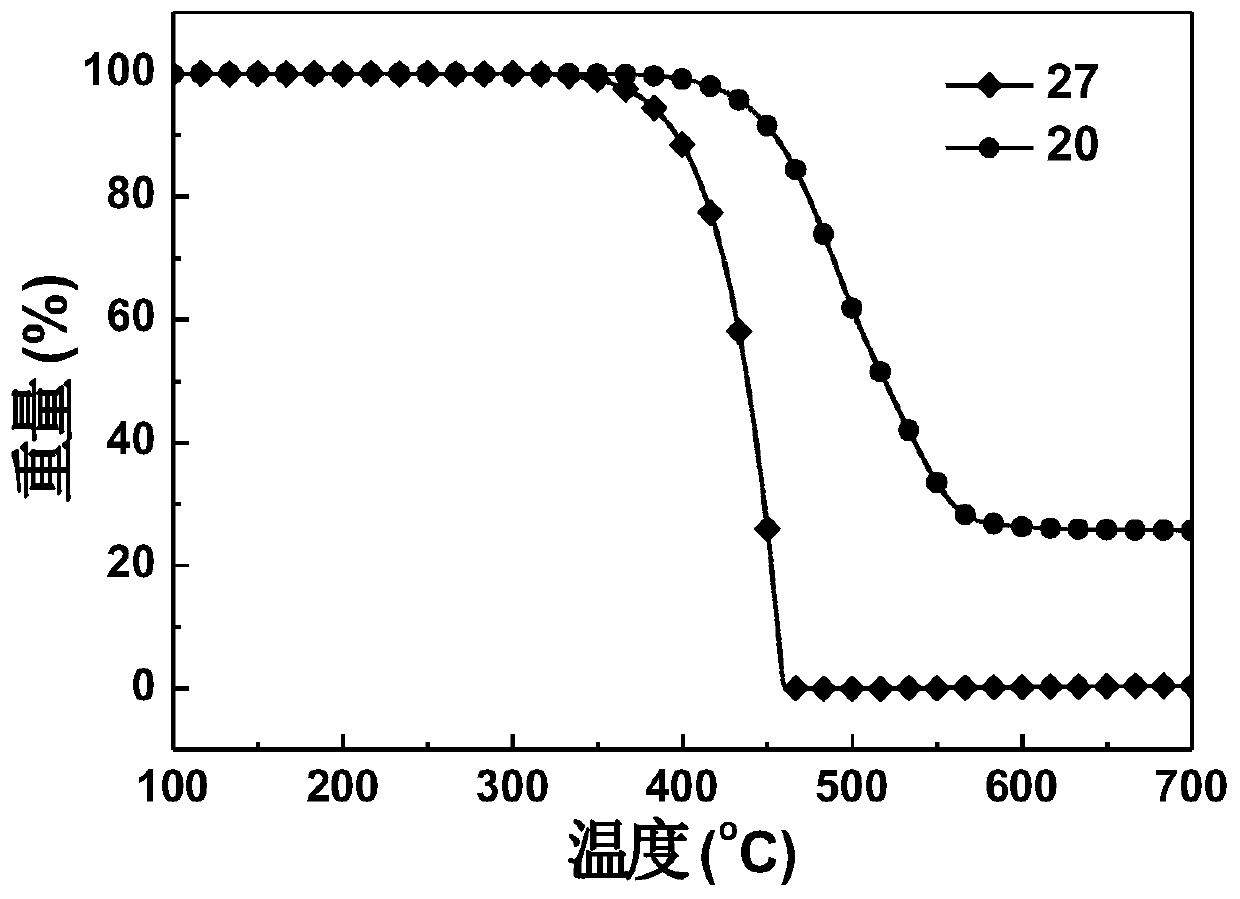
[0214] Embodiment 1: the synthesis of compound 20
[0215] (Synthesis of Intermediate 1)
[0216] The synthetic route of intermediate 1 is as follows:
[0217]
[0218] Under nitrogen, add 5.8g (47.8mmol) phenylboronic acid, 8.4g (79.6mmol) anhydrous sodium carbonate, 10.0g (39.8mmol) 2-bromo-3,5-difluoro Methyl benzoate, 470.8 mg (4.8 mmol) tetrakis(triphenylphosphine) palladium and 100 mL of a mixed solvent (toluene:water:ethanol=5:1:1 (V / V)). The system was gradually heated to reflux and reacted overnight under reflux. After the reaction was completed, the heating was stopped, and the reaction system was cooled to room temperature by itself. The reaction solution was poured into about 200 mL of water, and extracted with dichloromethane. The organic phase was dried with anhydrous sodium sulfate, concentrated under reduced pressure, and further purified by column chromatography (350 mesh silica gel, eluent: petroleum ether: dichloromethane = 20:1 (V / V)) to obtain light...
Embodiment 2
[0236] Embodiment 2: the synthesis of compound 27
[0237] (Synthesis of Intermediate 27A)
[0238] The synthetic route of intermediate 27A is shown below:
[0239]
[0240] Add 5g (23mmol) of intermediate 2 and 14.3g (50.6mmol) of 7,7-dimethyl-5,7-dihydroindeno[2,1-b]carbazole in a dry and clean 250mL three-neck flask and 12.7 g (92 mmol) of anhydrous potassium carbonate. The system was replaced with nitrogen back and forth three times to remove the air therein. Add 150mL of N-methylpyrrolidone, gradually raise the temperature to 180°C, and react at this temperature overnight. After the reaction was cooled, the inorganic salt was removed by suction filtration, and the filtrate was distilled under reduced pressure to obtain a reddish-brown residue. The crude product was further purified by column chromatography (350 mesh silica gel, eluent: petroleum ether:dichloromethane=4:1 (V / V)) to obtain 16.3 g of orange-red solid with a yield of 96%. MS (EI): m / z: 742.83 [M + ]....
Embodiment 3
[0249] Embodiment 3: the synthesis of compound 360
[0250] (Synthesis of Compound 360)
[0251] The synthetic route of compound 360 is as follows:
[0252]
[0253] Under nitrogen protection, 2.0 g (8.9 mmol) of 2-bromobiphenyl and 150 mL of anhydrous tetrahydrofuran were added to a dry and clean 250 mL three-neck flask, and stirred to dissolve at room temperature. The system was cooled to -78°C, and 3.9 mL (2.5 M, 9.8 mmol) of n-butyllithium was added dropwise at this temperature, and stirring was continued at this temperature for 1.5 h after the addition was complete. Subsequently, 6.1 g (8.1 mmol) of intermediate 20A was added in one batch, and the cooling bath was removed after the addition, and the reaction was warmed to room temperature by itself and continued to stir overnight. After the reaction, it was washed with water, dried, and spin-dried to obtain a white solid.
[0254] The above white solid was transferred to a 250mL one-necked bottle equipped with a ref...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com