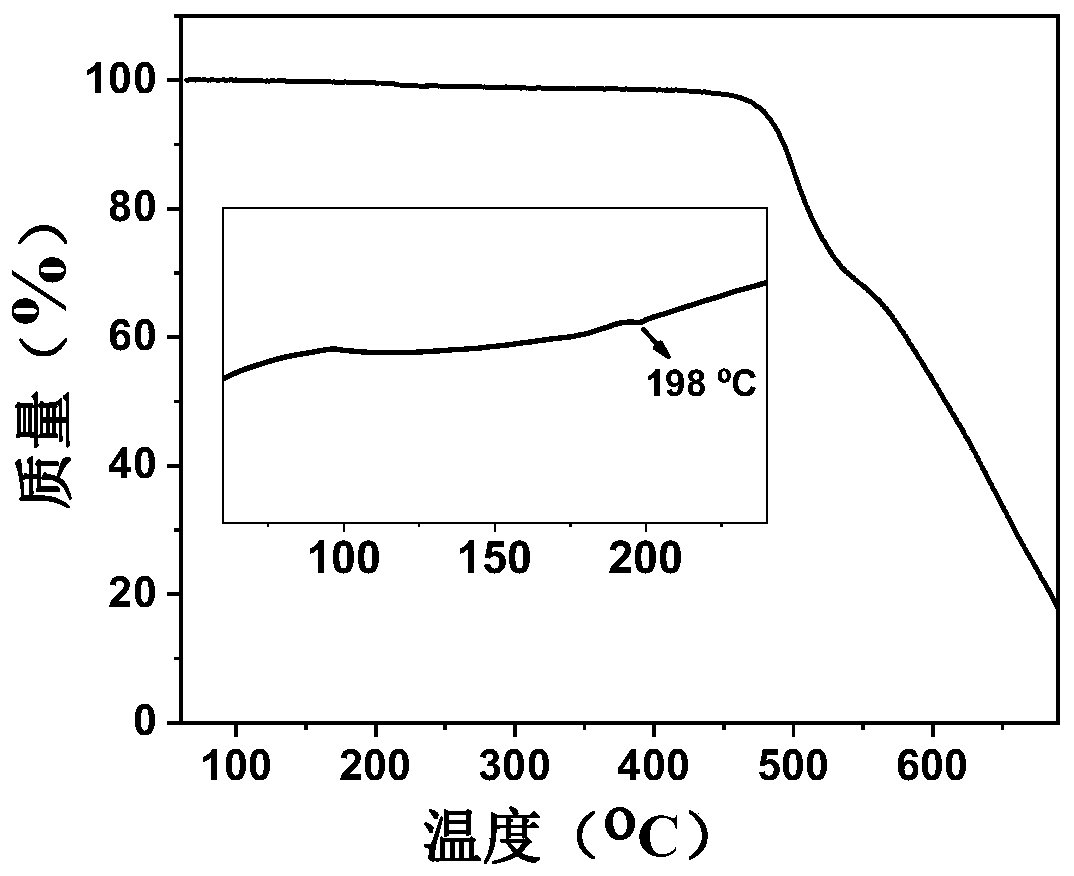
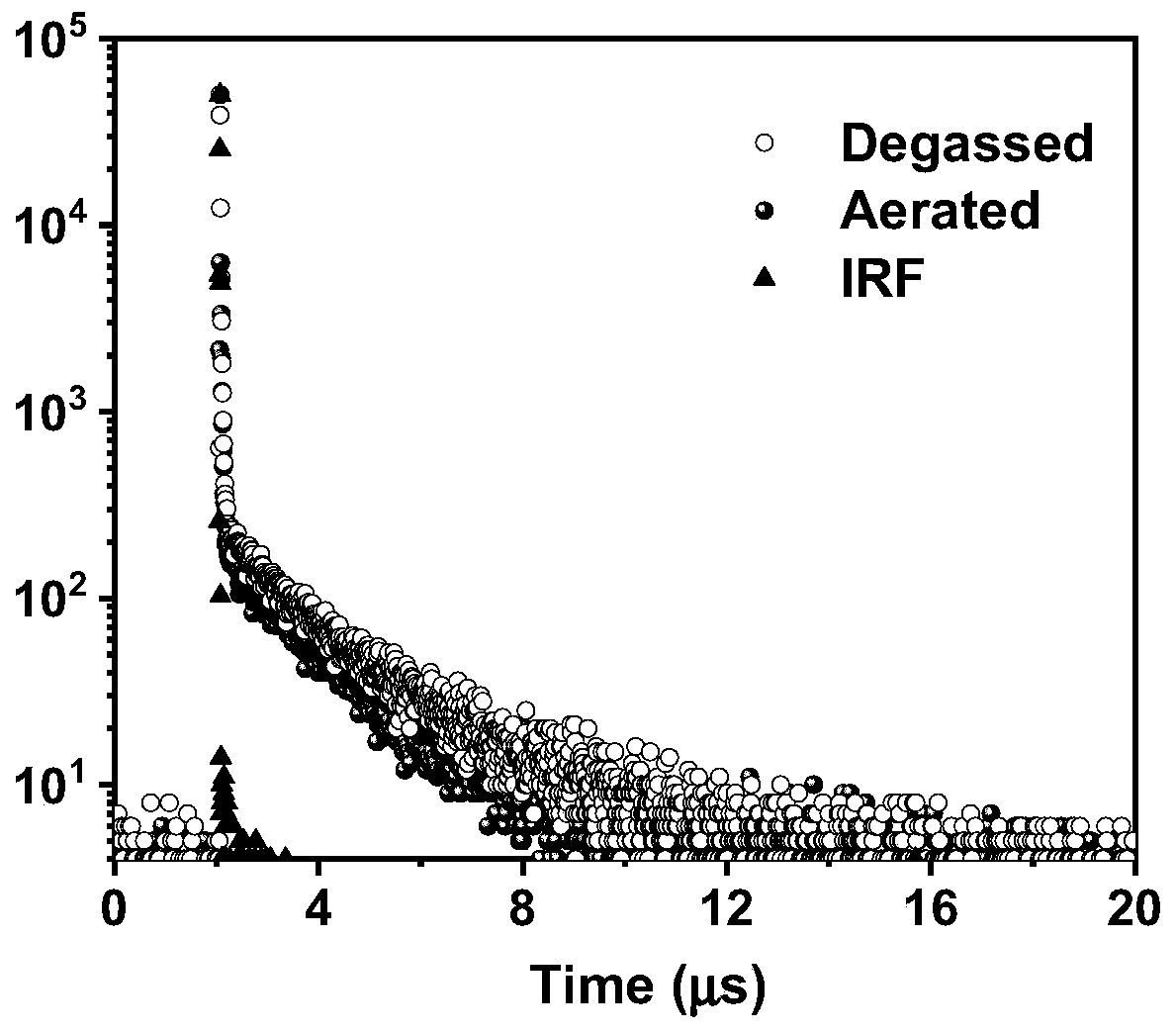
Star-shaped thermal activation delayed fluorescence material, electronic device and application thereof
A technology of thermally activated delayed and fluorescent materials, applied in luminescent materials, electric solid-state devices, material analysis by optical means, etc., can solve the problems of low efficiency and low thermal stability, reduce overlap, improve device efficiency, The effect of reducing the energy level difference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1: the preparation of compound 2:
[0064] (1) The preparation of intermediate 1-1, reaction formula is as follows:
[0065]
[0066] Add 1,3,5-triphenylbenzene (1.6g, 4.9mmol) and 4-fluorobenzenesulfonyl chloride (6.7g, 34mmol) into the two-necked flask, connect the condenser tube and connect it to the double-barrel tube, and communicate with argon gas protection. The flask was heated in an oil bath at 100°C for 5 minutes, then nitrobenzene (5 mL) was added, and anhydrous ferric chloride (0.56 g, 3.5 mmol) was added slowly, and heated and stirred at 100°C for 3 hours. The reaction solution was poured into 50 mL of methanol solution of hydrochloric acid (volume concentration 5%) and stirred vigorously, filtered, washed with 3% aqueous hydrochloric acid and deionized water, and dried in vacuo to obtain a brown crude solid. The crude product solid was dissolved in acetic acid, activated carbon was added to heat to reflux, and the acceptor intermediate 1-1 ...
Embodiment 2
[0070] Embodiment 2: the preparation of compound 60:
[0071] (1) The preparation of intermediate 2-1, the reaction formula is as follows:
[0072]
[0073] 1,3,5-tris(4-bromophenyl)benzene (500mg, 0.92mmol), (4-fluorophenyl)phenylphosphine (814mg, 3.7mmol), zinc powder (481mg, 7.4mmol), 2,2-bipyridine (115mg, 0.74mmol), nickel chloride hexahydrate (88mg, 0.37mmol) mixture was put into the two-necked bottle that is connected with condenser tube, added dimethylacetamide (20mL) under nitrogen protection, Stir at 150°C for 24 hours. After the reaction solution was cooled to room temperature, the mixture was poured into water and extracted with dichloromethane. The organic layer was washed with MgSO 4 After drying and filtering, the crude product was separated by silica gel chromatography (eluent: methanol, dichloromethane) to obtain 310 mg of product with a yield of 35%. MS(MALDI-TOF):m / z 961.2[M + ].
[0074] (2) The preparation of compound 60, the reaction formula is a...
Embodiment 3
[0076] Embodiment 3: the preparation of compound 78:
[0077] (1) The preparation of intermediate 3-1, the reaction formula is as follows:
[0078]
[0079] 1,4-Dibromobenzene (472mg, 2.0mmol) was dissolved in anhydrous ether (4mL), and cooled to -78°C under nitrogen protection. A solution of isobutyllithium (1.4M in cyclohexane, 1.43 mL, 4.410 mmol) was added slowly. Slowly warm to 0°C over 2 hours, then cool again to -78°C. The reaction solution was added dropwise to a solution of 4-tert-butyl-2-methylphenylboronic acid (288 mg, 1.5 mmol) in dry ether (5 mL) at -78°C. The reaction solution was stirred overnight at room temperature after slowly warming up. 1M aqueous hydrochloric acid (5 mL) was added to terminate the reaction, and stirred at room temperature for 15 minutes. The organic layer was collected, the aqueous layer was extracted three times with ether, all organic layers were combined, dried over anhydrous sodium sulfate and concentrated by rotary evaporation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com