Amantadine hapten, antigen, chemiluminiscence enzyme-linked immunoassay kit and application thereof
A technology of amantadine and hapten, applied in organic chemistry, biological testing, immunoglobulin, etc., can solve problems such as high equipment maintenance costs, high detection costs, and high technical requirements for operators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] The preparation of embodiment 1 amantadine hapten
[0071] 1. Preparation of amantadine hapten
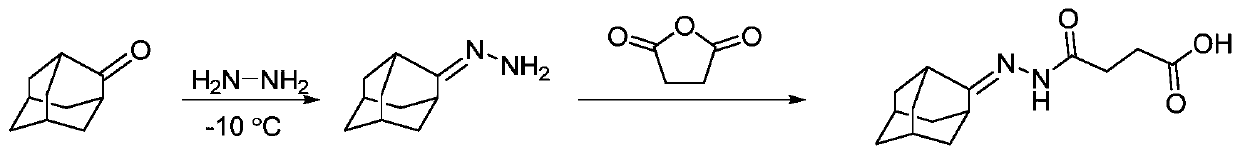
[0072] (1) Add 15.0g of 2-adamantanone and 6.3g of 80% hydrazine hydrate to 30mL of methanol solution, react at -10°C for 30min, evaporate the methanol solvent to obtain a transparent oily product, which is directly used without purification react in the next step.
[0073] (2) The above product was dissolved in 30 mL of chloroform, 10.12 g of triethylamine and 12.01 g of succinic anhydride, and after reflux and stirring for 1 h, the solvent was evaporated, and the residue was purified by column chromatography to obtain a white solid product, which was amantadine hapten. It was identified and successfully synthesized by mass spectrometry and nuclear magnetic resonance spectroscopy, and its spectral data are as follows: ESI-MS(+):265.155[M+H] + .
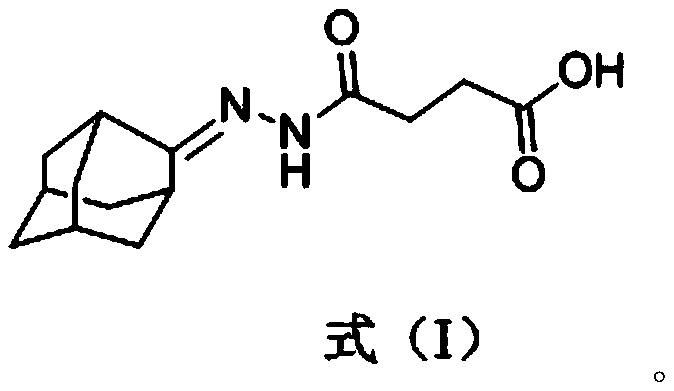
[0074] Its structural formula is shown in formula (I):
[0075]
[0076] The synthetic route of this amantadine hapten is...
Embodiment 2
[0077] Embodiment 2 Preparation of amantadine antigen, coating agent and specific antibody
[0078] 1. Synthesis of amantadine antigen
[0079] (1) Weigh 6.12 mg of the amantadine hapten prepared in Example 1, dissolve it in 1 mL of N,N-dimethylformamide (DMF), add 4.3 mg of 1-(3-dimethylaminopropyl) -3-Ethylcarbodiimide hydrochloride and 5.2 mg of N-hydroxysuccinimide were reacted with stirring at room temperature for 2 hours to obtain solution C;
[0080] (2) 50 mg of BSA was dissolved in 5 mL of 0.1 mol / L sodium bicarbonate buffer to obtain a protein solution;
[0081] (3) Add the above solution C to the protein solution drop by drop, stir overnight at room temperature, dialyze with PBS solution at 4°C for 3 days, and change the dialysate 6 times during the period; Filter, aliquot into ampoules, and store at -20°C.
[0082] This amantadine antigen was used as the amantadine immunogen.
[0083] 2. Synthesis of Amantadine Coating Source
[0084] The method is the same as...
Embodiment 3
[0104] The preparation of the chemiluminescent ELISA kit of embodiment 3 amantadine
[0105] 1. Prepare reagents:
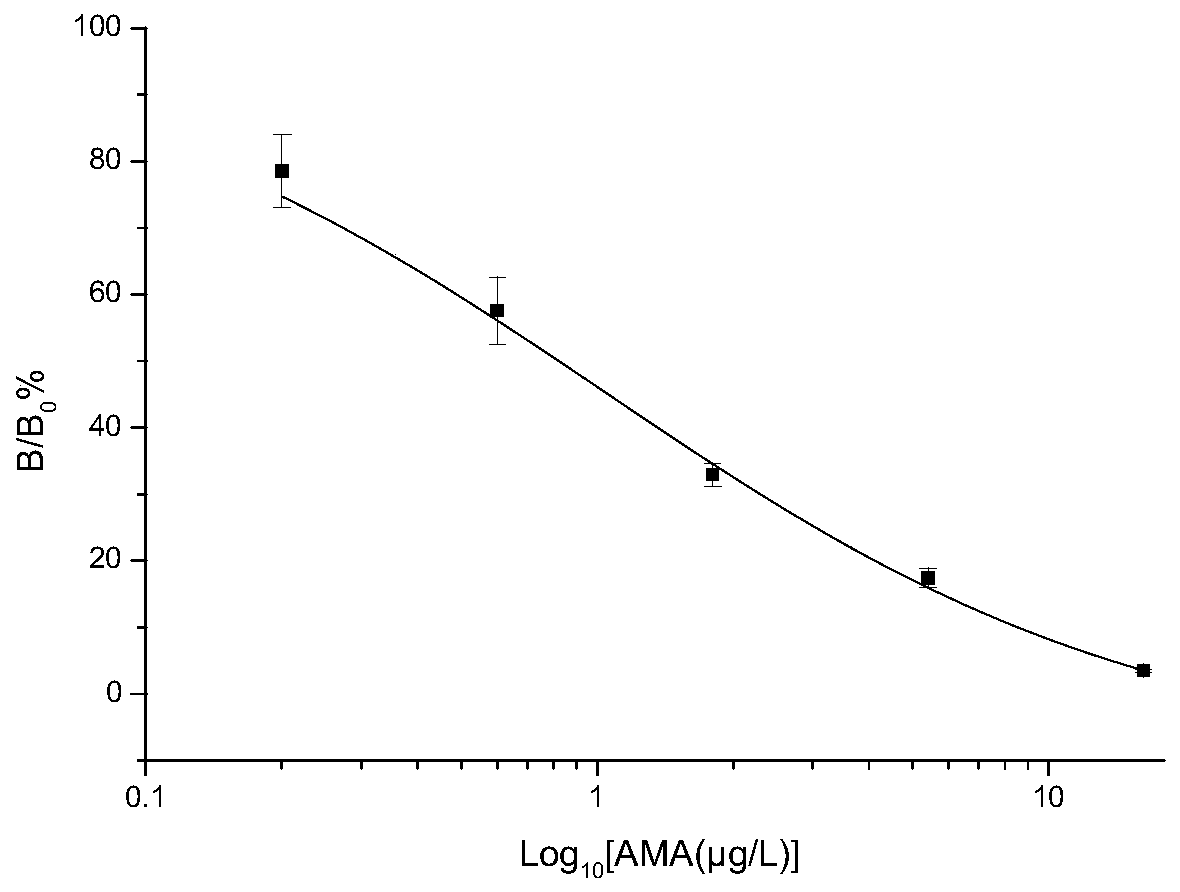
[0106] (1) ELISA plate coated with amantadine antigen: 96-well detachable ELISA plate, coated with amantadine antigen and blocking solution, the coating concentration is 65.5 μg / L. The amantadine antigen is a conjugate of amantadine hapten and BSA.
[0107] Dilute the coated antigen to 65.5 μg / L with coating solution, add 150 μL of coating solution to each well, incubate overnight at 37°C, pour off the liquid in the well, wash twice with washing solution, and pat dry. Then add 170 μL of blocking solution to each well, incubate at 37°C for 2 hours, pour off the liquid in the well, dry in an oven at 37°C, and store in a vacuum-sealed aluminum foil bag at 4°C.
[0108] (2) Preparation of amantadine standard solution: Accurately weigh amantadine standard solution, dilute to 1.00mg / mL with chromatographic grade methanol, and then use 0.04mol / L PB buffer solution (fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com