Medical bleeding stopping dressing, preparation method and application of preparation method
A technology of hemostatic dressings and raw materials, applied in medical formula, medical science, bandages, etc., can solve the problems of increasing pain and economic burden of patients, secondary injury of wounds, and large loss of drugs, so as to reduce the chance of wound infection and improve Biocompatibility, effect of reducing labor intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The invention provides a kind of preparation method of medical hemostatic dressing, it comprises the steps:
[0056] Dissolving the recombinant collagen to obtain A solution;
[0057] Dissolving the water-soluble high molecular polymer and the moisture retaining agent into the solution to obtain the B solution;
[0058] A solution, B solution, an optional stabilizer and an optional plasticizer are mixed to obtain a first mixed solution;
[0059] The first mixed solution is frozen, then soaked, washed, centrifuged and sterilized, wherein, in parts by weight, the medical hemostatic dressing contains 0.5-2 parts of recombinant collagen, 1-5 parts of water-soluble polymer substance and 0.2-1 part of moisture retaining agent.
[0060] Wherein, "optionally" means that the raw material may or may not be added.
[0061] In a preferred embodiment of the present invention, wherein the method comprises the following steps:
[0062] Dissolving the recombinant collagen to form A...
Embodiment 1
[0082] The preparation of embodiment 1 medical hemostatic dressing
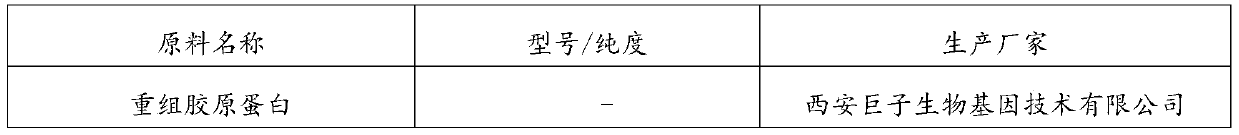
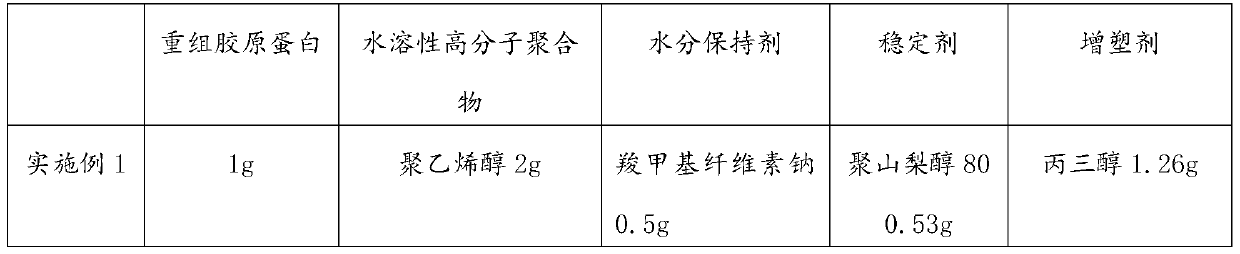
[0083] (1) Dissolve 1 g of recombinant collagen in 20 mL of distilled water at room temperature to form A solution;
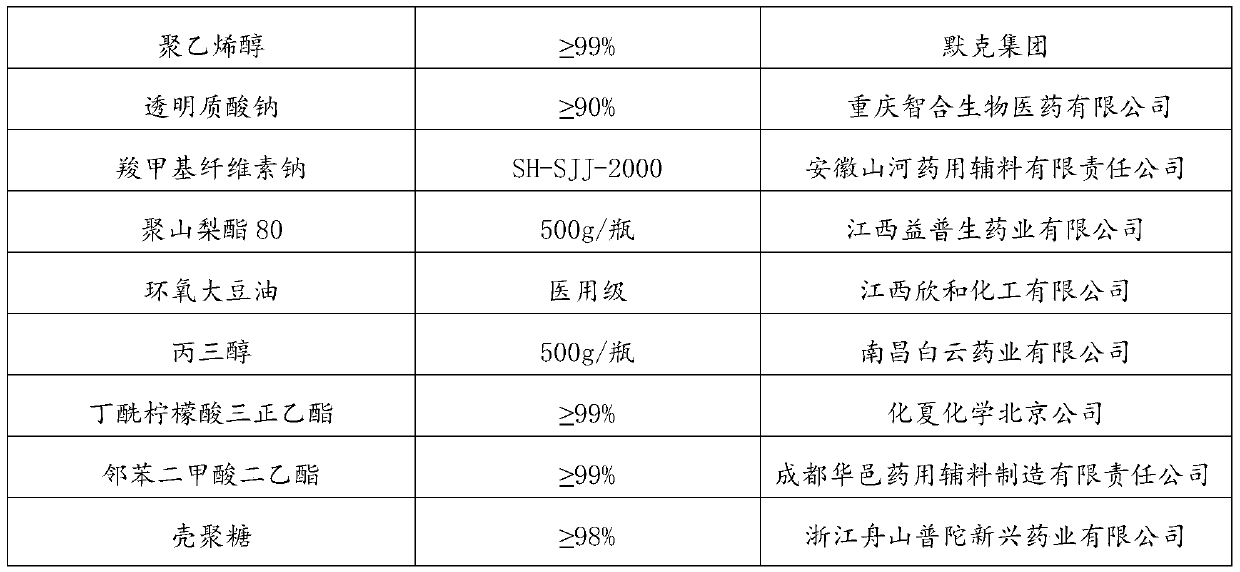
[0084] (2) Dissolve 2g of polyvinyl alcohol and 0.5g of sodium carboxymethylcellulose in 28.5mL of distilled water in a water bath at 80°C to form solution B;
[0085] (3) Mix 20mL of LA solution, 28.5mL of B solution, 1.26g of glycerol, and 0.53g of polysorbate 80 in a 60°C water bath, take it out of the water bath and immediately pour it into an aluminum mold to obtain the first mixed solution.
[0086] (4) Freeze the first mixed solution in a -20°C refrigerator for 24 hours, take it out and place it at room temperature for 3 hours to fully thaw, repeat the freezing-thawing process 3 times, and obtain the second mixed solution;
[0087] (5) Soak the second mixed solution in purified water for 2 hours, then wash it in an ultrasonic cleaning machine for 3 times, each time for 10 minutes to ob...
Embodiment 2
[0089] The preparation of embodiment 2 medical hemostatic dressings
[0090] (1) Dissolve 2g of recombinant collagen in 20mL of distilled water at room temperature to form A solution;
[0091] (2) Dissolve 1g of sodium hyaluronate and 0.5g of sodium carboxymethylcellulose in 29mL of distilled water in a water bath at 80°C to form solution B;
[0092] (3) Fully mix 20mL of LA solution, 29mL of BB solution, 0.8g of butyryl tri-n-hexyl citrate, and 0.9g of epoxidized soybean oil in a 60°C water bath, take it out of the water bath and immediately pour it into an aluminum mold to obtain the first mixture solution;
[0093] (4) The first mixed solution was frozen in a -20°C refrigerator for 24 hours, then taken out and placed at room temperature for 3 hours to fully thaw, and the freezing-thawing process was repeated 3 times to obtain the second mixed solution;
[0094] (5) Soak the second mixed solution in purified water for 3 hours, then wash it in an ultrasonic cleaning machine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com